Triage process for the assessment of coronavirus disease 2019-positive patients with cancer: The ONCOVID prospective study
- PMID: 33270908
- PMCID: PMC7753308
- DOI: 10.1002/cncr.33366
Triage process for the assessment of coronavirus disease 2019-positive patients with cancer: The ONCOVID prospective study
Abstract
Background: Patients with cancer are considered at high risk for the novel respiratory illness coronavirus disease 2019 (COVID-19). General measures to keep COVID-19-free cancer divisions have been adopted worldwide. The objective of this study was to evaluate the efficacy of triage to identify COVID-19 among patients with cancer.
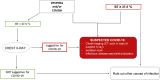
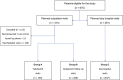
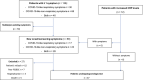
Methods: From March 20 to April 17, 2020, data were collected from patients who were treated or followed at the authors' institution in a prospective clinical trial. The primary endpoint was to estimate the cumulative incidence of COVID-19-positive patients who were identified using a triage process through the aid of medical and patient questionnaires. Based on a diagnostic algorithm, patients with suspect symptoms underwent an infectious disease specialist's evaluation and a COVID-19 swab. Serologic tests were proposed for patients who had symptoms or altered laboratory tests that did not fall into the diagnostic algorithm but were suspicious for COVID-19.
Results: Overall, 562 patients were enrolled. Six patients (1%) were diagnosed with COVID-19, of whom 4 (67%) had the disease detected through telehealth triage, and 2 patients (33%) without suspect symptoms at triage had the disease detected later. Seventy-one patients (13%) had suspect symptoms and/or altered laboratory tests that were not included in the diagnostic algorithm and, of these, 47 patients (73%) underwent testing for severe acute respiratory syndrome coronavirus 2 antibody: 6 (13%) were positive for IgG (n = 5) or for both IgM and IgG (n = 1), and antibody tests were negative in the remaining 41 patients.
Conclusions: The triage process had a positive effect on the detection of COVID-19 in patients with cancer. Telehealth triage was helpful in detecting suspect patients and to keep a COVID-19-free cancer center. The overall incidence of COVID-19 diagnosis (1%) and antibody positivity (13%) in patients with suspect symptoms was similar to that observed in the general population.
Keywords: COVID-19 antibody; COVID-19 infection; coronavirus disease 2019 (COVID-19); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); triage.
© 2020 American Cancer Society.
Conflict of interest statement
The authors made no disclosures.
Figures
References
-
- World Health Organization (WHO) . Coronavirus disease (COVID‐19) pandemic, update: June 25, 2020. WHO; 2020. Accessed August 27, 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019

