Blood-based biomarkers of human papillomavirus-associated cancers: A systematic review and meta-analysis
- PMID: 33270909
- PMCID: PMC8135101
- DOI: 10.1002/cncr.33221
Blood-based biomarkers of human papillomavirus-associated cancers: A systematic review and meta-analysis
Abstract
Background: Despite the significant societal burden of human papillomavirus (HPV)-associated cancers, clinical screening interventions for HPV-associated noncervical cancers are not available. Blood-based biomarkers may help close this gap in care.
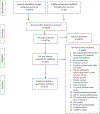
Methods: Five databases were searched, 5687 articles were identified, and 3631 unique candidate titles and abstracts were independently reviewed by 2 authors; 702 articles underwent a full-text review. Eligibility criteria included the assessment of a blood-based biomarker within a cohort or case-control study.
Results: One hundred thirty-seven studies were included. Among all biomarkers assessed, HPV-16 E seropositivity and circulating HPV DNA were most significantly correlated with HPV-associated cancers in comparison with cancer-free controls. In most scenarios, HPV-16 E6 seropositivity varied nonsignificantly according to tumor type, specimen collection timing, and anatomic site (crude odds ratio [cOR] for p16+ or HPV+ oropharyngeal cancer [OPC], 133.10; 95% confidence interval [CI], 59.40-298.21; cOR for HPV-unspecified OPC, 25.41; 95% CI, 8.71-74.06; cOR for prediagnostic HPV-unspecified OPC, 59.00; 95% CI, 15.39-226.25; cOR for HPV-unspecified cervical cancer, 12.05; 95% CI, 3.23-44.97; cOR for HPV-unspecified anal cancer, 73.60; 95% CI, 19.68-275.33; cOR for HPV-unspecified penile cancer, 16.25; 95% CI, 2.83-93.48). Circulating HPV-16 DNA was a valid biomarker for cervical cancer (cOR, 15.72; 95% CI, 3.41-72.57). In 3 cervical cancer case-control studies, cases exhibited unique microRNA expression profiles in comparison with controls. Other assessed biomarker candidates were not valid.
Conclusions: HPV-16 E6 antibodies and circulating HPV-16 DNA are the most robustly analyzed and most promising blood-based biomarkers for HPV-associated cancers to date. Comparative validity analyses are warranted. Variations in tumor type-specific, high-risk HPV DNA prevalence according to anatomic site and world region highlight the need for biomarkers targeting more high-risk HPV types. Further investigation of blood-based microRNA expression profiling appears indicated.
Keywords: anal cancer; biomarker; blood biomarker; cancer prevention; cancer surveillance; cervical cancer; human papillomavirus (HPV); oropharyngeal cancer; penile cancer; screening.
© 2020 American Cancer Society.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES
The other authors made no disclosures.
Figures

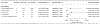

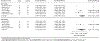

References
-
- Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus–associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661–666. - PubMed
-
- American Cancer Society. Cancer Facts & Figures 2018. American Cancer Society; 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

