Cryo-EM Structures of SARS-CoV-2 Spike without and with ACE2 Reveal a pH-Dependent Switch to Mediate Endosomal Positioning of Receptor-Binding Domains
- PMID: 33271067
- PMCID: PMC7670890
- DOI: 10.1016/j.chom.2020.11.004
Cryo-EM Structures of SARS-CoV-2 Spike without and with ACE2 Reveal a pH-Dependent Switch to Mediate Endosomal Positioning of Receptor-Binding Domains
Abstract
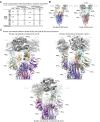
The SARS-CoV-2 spike employs mobile receptor-binding domains (RBDs) to engage the human ACE2 receptor and to facilitate virus entry, which can occur through low-pH-endosomal pathways. To understand how ACE2 binding and low pH affect spike conformation, we determined cryo-electron microscopy structures-at serological and endosomal pH-delineating spike recognition of up to three ACE2 molecules. RBDs freely adopted "up" conformations required for ACE2 interaction, primarily through RBD movement combined with smaller alterations in neighboring domains. In the absence of ACE2, single-RBD-up conformations dominated at pH 5.5, resolving into a solitary all-down conformation at lower pH. Notably, a pH-dependent refolding region (residues 824-858) at the spike-interdomain interface displayed dramatic structural rearrangements and mediated RBD positioning through coordinated movements of the entire trimer apex. These structures provide a foundation for understanding prefusion-spike mechanics governing endosomal entry; we suggest that the low pH all-down conformation potentially facilitates immune evasion from RBD-up binding antibody.
Keywords: ACE2 receptor; COVID-19; endosomal entry; pH-dependent switch; receptor-binding domain (RBD); structural rearrangement; type 1 fusion machine.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures






Update of
-
Cryo-EM Structures Delineate a pH-Dependent Switch that Mediates Endosomal Positioning of SARS-CoV-2 Spike Receptor-Binding Domains.bioRxiv [Preprint]. 2020 Jul 31:2020.07.04.187989. doi: 10.1101/2020.07.04.187989. bioRxiv. 2020. Update in: Cell Host Microbe. 2020 Dec 9;28(6):867-879.e5. doi: 10.1016/j.chom.2020.11.004. PMID: 32637958 Free PMC article. Updated. Preprint.
References
-
- Benjaminsen R.V., Sun H., Henriksen J.R., Christensen N.M., Almdal K., Andresen T.L. Evaluating nanoparticle sensor design for intracellular pH measurements. ACS Nano. 2011;5:5864–5873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

