Chest CT in COVID-19 at the ED: Validation of the COVID-19 Reporting and Data System (CO-RADS) and CT Severity Score: A Prospective, Multicenter, Observational Study
- PMID: 33271157
- PMCID: PMC7704067
- DOI: 10.1016/j.chest.2020.11.026
Chest CT in COVID-19 at the ED: Validation of the COVID-19 Reporting and Data System (CO-RADS) and CT Severity Score: A Prospective, Multicenter, Observational Study
Abstract
Background: CT is thought to play a key role in coronavirus disease 2019 (COVID-19) diagnostic workup. The possibility of comparing data across different settings depends on the systematic and reproducible manner in which the scans are analyzed and reported. The COVID-19 Reporting and Data System (CO-RADS) and the corresponding CT severity score (CTSS) introduced by the Radiological Society of the Netherlands (NVvR) attempt to do so. However, this system has not been externally validated.
Research question: We aimed to prospectively validate the CO-RADS as a COVID-19 diagnostic tool at the ED and to evaluate whether the CTSS is associated with prognosis.
Study design and methods: We conducted a prospective, observational study in two tertiary centers in The Netherlands, between March 19 and May 28, 2020. We consecutively included 741 adult patients at the ED with suspected COVID-19, who received a chest CT and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR (PCR). Diagnostic accuracy measures were calculated for CO-RADS, using PCR as reference. Logistic regression was performed for CTSS in relation to hospital admission, ICU admission, and 30-day mortality.
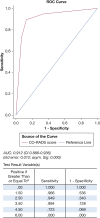
Results: Seven hundred forty-one patients were included. We found an area under the curve (AUC) of 0.91 (CI, 0.89-0.94) for CO-RADS using PCR as reference. The optimal CO-RADS cutoff was 4, with a sensitivity of 89.4% (CI, 84.7-93.0) and specificity of 87.2% (CI, 83.9-89.9). We found a significant association between CTSS and hospital admission, ICU admission, and 30-day mortality; adjusted ORs per point increase in CTSS were 1.19 (CI, 1.09-1.28), 1.23 (1.15-1.32), 1.14 (1.07-1.22), respectively. Intraclass correlation coefficients for CO-RADS and CTSS were 0.94 (0.91-0.96) and 0.82 (0.70-0.90).
Interpretation: Our findings support the use of CO-RADS and CTSS in triage, diagnosis, and management decisions for patients presenting with possible COVID-19 at the ED.
Keywords: COVID-19; CT; emergency medicine; pneumonia.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
The Potential Role for CT in the Diagnosis of Coronavirus Disease 2019.Chest. 2021 Mar;159(3):906-907. doi: 10.1016/j.chest.2020.12.018. Chest. 2021. PMID: 33678273 Free PMC article. No abstract available.
References
-
- Coronavirus Update (Live)—Worldometers. https://www.worldometers.info/coronavirus/ Accessed February 2, 2021.
-
- Johns Hopkins Coronavirus Resource Center COVID-19 Map. https://coronavirus.jhu.edu/map.html Accessed February 2, 2021.
-
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 323(22):2249-2251. - PubMed
-
- Abbasi J. The promise and peril of antibody testing for COVID-19. JAMA. 2020;323(19):1881–1883. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

