A highly effective reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the rapid detection of SARS-CoV-2 infection
- PMID: 33271166
- PMCID: PMC7703389
- DOI: 10.1016/j.jinf.2020.10.039
A highly effective reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the rapid detection of SARS-CoV-2 infection
Abstract
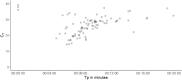
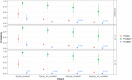
The COVID-19 pandemic has illustrated the importance of simple, rapid and accurate diagnostic testing. This study describes the validation of a new rapid SARS-CoV-2 RT-LAMP assay for use on extracted RNA or directly from swab offering an alternative diagnostic pathway that does not rely on traditional reagents that are often in short supply during a pandemic. Analytical specificity (ASp) of this new RT-LAMP assay was 100% and analytical sensitivity (ASe) was between 1 × 101 and 1 × 102 copies per reaction when using a synthetic DNA target. The overall diagnostic sensitivity (DSe) and specificity (DSp) of RNA RT-LAMP was 97% and 99% respectively, relative to the standard of care rRT-PCR. When a CT cut-off of 33 was employed, above which increasingly evidence suggests there is a low risk of patients shedding infectious virus, the diagnostic sensitivity was 100%. The DSe and DSp of Direct RT-LAMP (that does not require RNA extraction) was 67% and 97%, respectively. When setting CT cut-offs of ≤33 and ≤25, the DSe increased to 75% and 100%, respectively, time from swab-to-result, CT < 25, was < 15 min. We propose that RNA RT-LAMP could replace rRT-PCR where there is a need for increased sample throughput and Direct RT-LAMP as a near-patient screening tool to rapidly identify highly contagious individuals within emergency departments and care homes during times of increased disease prevalence ensuring negative results still get laboratory confirmation.
Keywords: COVID-19; Direct RNA detection; Near patient testing; RT-LAMP; Rapid diagnostics; SARS-CoV-2.
Crown Copyright © 2020. Published by Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Home-based SARS-CoV-2 lateral flow antigen testing in hospital workers.J Infect. 2021 Feb;82(2):282-327. doi: 10.1016/j.jinf.2021.01.008. Epub 2021 Feb 8. J Infect. 2021. PMID: 33573777 Free PMC article. No abstract available.
-
Critical evaluation of the methodology used by Wilson-Davies et al., (2020) entitled "Concerning the Optigene Direct LAMP assay, and it`s use in at-risk groups and hospital staff".J Infect. 2021 Feb;82(2):282-327. doi: 10.1016/j.jinf.2021.01.012. Epub 2021 Feb 8. J Infect. 2021. PMID: 33573778 No abstract available.
-
Concerning the OptiGene Direct LAMP assay, and it’s use in at-risk groups and hospital staff.J Infect. 2021 Feb;82(2):282-327. doi: 10.1016/j.jinf.2021.01.013. Epub 2021 Feb 8. J Infect. 2021. PMID: 33573779 Free PMC article. No abstract available.
-
Use of lateral flow devices allows rapid triage of patients with SARS-CoV-2 on admission to hospital.J Infect. 2021 Jun;82(6):276-316. doi: 10.1016/j.jinf.2021.02.025. Epub 2021 Mar 1. J Infect. 2021. PMID: 33662408 Free PMC article. No abstract available.
-
Quantitative SARS-CoV-2 antigen test as a tool able to predict the stage of the infection.J Infect. 2022 Mar;84(3):418-467. doi: 10.1016/j.jinf.2021.12.013. Epub 2021 Dec 22. J Infect. 2022. PMID: 34953907 Free PMC article.
References
-
- WHO. Pneumonia of unknown cause. Available at https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-ch... 2020.
-
- Gorbalenya Alexander E., Baker Susan C., Baric Ralph S., De G.R.J, Gulyaeva Anastasia A., Haagmans Bart L. The species and its viruses – a statement of the Coronavirus Study Group. Biorxiv Cold Spring Harb Lab. 2020:1–15. doi: 10.1101/2020.02.07.937862. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

