Protective Effect of Shikimic Acid against Cisplatin-Induced Renal Injury: In Vitro and In Vivo Studies
- PMID: 33271750
- PMCID: PMC7759863
- DOI: 10.3390/plants9121681
Protective Effect of Shikimic Acid against Cisplatin-Induced Renal Injury: In Vitro and In Vivo Studies
Abstract
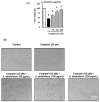
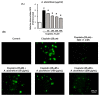
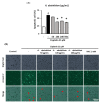
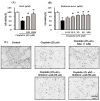
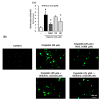
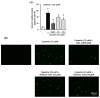
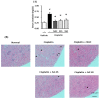
Nephrotoxicity is a serious side effect of cisplatin, which is one of the most frequently used drugs for cancer treatment. This study aimed to assess the renoprotective effect of Artemisia absinthium extract and its bioactive compound (shikimic acid) against cisplatin-induced renal injury. An in vitro assay was performed in kidney tubular epithelial cells (LLC-PK1) with 50, 100, and 200 µg/mL A. absinthium extract and 25 and 50 µM shikimic acid, and cytotoxicity was induced by 25 µM cisplatin. BALB/c mice (6 weeks old) were injected with 16 mg/kg cisplatin once and orally administered 25 and 50 mg/kg shikimic acid daily for 4 days. The results showed that the A. absinthium extract reversed the decrease in renal cell viability induced by cisplatin, whereas it decreased the reactive oxidative stress accumulation and apoptosis in LLC-PK1 cells. Shikimic acid also reversed the effect on cell viability but decreased oxidative stress and apoptosis in renal cells compared with the levels in the cisplatin-treated group. Furthermore, shikimic acid protected against kidney injury in cisplatin-treated mice by reducing serum creatinine levels. The protective effect of shikimic acid against cisplatin-mediated kidney injury was confirmed by the recovery of histological kidney injury in cisplatin-treated mice. To the best of our knowledge, this study is the first report on the nephroprotective effect of A. absinthium extract and its mechanism of action against cisplatin-induced renal injury.
Keywords: Artemisia absinthium; apoptosis; cisplatin; nephrotoxicity; shikimic acid.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Protective Effect of Artemisia asiatica Extract and Its Active Compound Eupatilin against Cisplatin-Induced Renal Damage.Evid Based Complement Alternat Med. 2015;2015:483980. doi: 10.1155/2015/483980. Epub 2015 Oct 11. Evid Based Complement Alternat Med. 2015. PMID: 26539226 Free PMC article.
-
Protective Effect of Panduratin A on Cisplatin-Induced Apoptosis of Human Renal Proximal Tubular Cells and Acute Kidney Injury in Mice.Biol Pharm Bull. 2021;44(6):830-837. doi: 10.1248/bpb.b21-00036. Biol Pharm Bull. 2021. PMID: 34078815
-
Protective effects of 6-hydroxy-1-methylindole-3-acetonitrile on cisplatin-induced oxidative nephrotoxicity via Nrf2 inactivation.Food Chem Toxicol. 2013 Dec;62:159-66. doi: 10.1016/j.fct.2013.08.039. Epub 2013 Aug 26. Food Chem Toxicol. 2013. PMID: 23989062
-
In Vitro and In Vivo Nephroprotective Effects of Nelumbo nucifera Seedpod Extract against Cisplatin-Induced Renal Injury.Plants (Basel). 2022 Dec 2;11(23):3357. doi: 10.3390/plants11233357. Plants (Basel). 2022. PMID: 36501396 Free PMC article.
-
Phytochemical profiling and nephroprotective potential of ethanolic leaf extract of Polyalthia longifolia against cisplatin-induced oxidative stress in rat model.J Ethnopharmacol. 2024 May 23;326:117922. doi: 10.1016/j.jep.2024.117922. Epub 2024 Feb 24. J Ethnopharmacol. 2024. PMID: 38403004
Cited by
-
Mechanism and Protective Effect of Smilax glabra Roxb on the Treatment of Heart Failure via Network Pharmacology Analysis and Vitro Verification.Front Pharmacol. 2022 May 23;13:868680. doi: 10.3389/fphar.2022.868680. eCollection 2022. Front Pharmacol. 2022. PMID: 35677443 Free PMC article.
-
Shikimic acid recovers diarrhea and its complications in SD rats fed lactose diet to induce diarrhea.Lab Anim Res. 2023 Nov 10;39(1):28. doi: 10.1186/s42826-023-00179-y. Lab Anim Res. 2023. PMID: 37950334 Free PMC article.
-
Remodeling of tumour microenvironment: strategies to overcome therapeutic resistance and innovate immunoengineering in triple-negative breast cancer.Front Immunol. 2024 Dec 10;15:1455211. doi: 10.3389/fimmu.2024.1455211. eCollection 2024. Front Immunol. 2024. PMID: 39720730 Free PMC article. Review.
-
Protective effects of shikimic acid against thioacetamide-induced hepatic fibrosis: role of Nrf2/NF-κB signaling pathways.Front Pharmacol. 2025 May 30;16:1609711. doi: 10.3389/fphar.2025.1609711. eCollection 2025. Front Pharmacol. 2025. PMID: 40520202 Free PMC article.
-
Therapeutic strategies for hypertension: exploring the role of microbiota-derived short-chain fatty acids in kidney physiology and development.Pediatr Nephrol. 2025 Jul 10. doi: 10.1007/s00467-025-06883-2. Online ahead of print. Pediatr Nephrol. 2025. PMID: 40637840 Review.
References
-
- Rosenberg B., Vancamp L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970;30:1799–1802. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

