The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy
- PMID: 33271772
- PMCID: PMC7760327
- DOI: 10.3390/cancers12123594
The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy
Abstract
In the human body, copper (Cu) is a major and essential player in a large number of cellular mechanisms and signaling pathways. The involvement of Cu in oxidation-reduction reactions requires close regulation of copper metabolism in order to avoid toxic effects. In many types of cancer, variations in copper protein levels have been demonstrated. These variations result in increased concentrations of intratumoral Cu and alterations in the systemic distribution of copper. Such alterations in Cu homeostasis may promote tumor growth or invasiveness or may even confer resistance to treatments. Once characterized, the dysregulated Cu metabolism is pinpointing several promising biomarkers for clinical use with prognostic or predictive capabilities. The altered Cu metabolism in cancer cells and the different responses of tumor cells to Cu are strongly supporting the development of treatments to disrupt, deplete, or increase Cu levels in tumors. The metallic nature of Cu as a chemical element is key for the development of anticancer agents via the synthesis of nanoparticles or copper-based complexes with antineoplastic properties for therapy. Finally, some of these new therapeutic strategies such as chelators or ionophores have shown promising results in a preclinical setting, and others are already in the clinic.
Keywords: cancer; copper homeostasis; diagnostic; prognostic; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
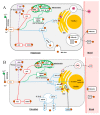
References
-
- Chen G.F., Sudhahar V., Youn S.W., Das A., Cho J., Kamiya T., Urao N., McKinney R.D., Surenkhuu B., Hamakubo T., et al. Copper Transport Protein Antioxidant-1 Promotes Inflammatory Neovascularization via Chaperone and Transcription Factor Function. Sci. Rep. 2015;5:14780. doi: 10.1038/srep14780. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

