Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance
- PMID: 33271787
- PMCID: PMC7730664
- DOI: 10.3390/molecules25235662
Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance
Abstract
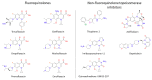
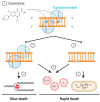
Fluoroquinolones (FQs) are arguably among the most successful antibiotics of recent times. They have enjoyed over 30 years of clinical usage and become essential tools in the armoury of clinical treatments. FQs target the bacterial enzymes DNA gyrase and DNA topoisomerase IV, where they stabilise a covalent enzyme-DNA complex in which the DNA is cleaved in both strands. This leads to cell death and turns out to be a very effective way of killing bacteria. However, resistance to FQs is increasingly problematic, and alternative compounds are urgently needed. Here, we review the mechanisms of action of FQs and discuss the potential pathways leading to cell death. We also discuss quinolone resistance and how quinolone treatment can lead to resistance to non-quinolone antibiotics.
Keywords: DNA gyrase; DNA topology; antibacterials; antibiotic resistance; fluoroquinolones; supercoiling; topoisomerases.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of this study or the decision to publish.
Figures





References
-
- Oliphant C.M., Green G.M. Quinolones: A comprehensive review. Am. Fam. Physician. 2002;65:455–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

