Protein Carbonylation and Lipid Peroxidation in Hematological Malignancies
- PMID: 33271863
- PMCID: PMC7761105
- DOI: 10.3390/antiox9121212
Protein Carbonylation and Lipid Peroxidation in Hematological Malignancies
Abstract
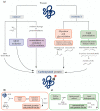
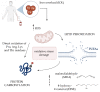
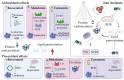
Among the different mechanisms involved in oxidative stress, protein carbonylation and lipid peroxidation are both important modifications associated with the pathogenesis of several diseases, including cancer. Hematopoietic cells are particularly vulnerable to oxidative damage, as the excessive production of reactive oxygen species and associated lipid peroxidation suppress self-renewal and induce DNA damage and genomic instability, which can trigger malignancy. A richer understanding of the clinical effects of oxidative stress might improve the prognosis of these diseases and inform therapeutic strategies. The most common protein carbonylation and lipid peroxidation compounds, including hydroxynonenal, malondialdehyde, and advanced oxidation protein products, have been investigated for their potential effect on hematopoietic cells in several studies. In this review, we focus on the most important protein carbonylation and lipid peroxidation biomarkers in hematological malignancies, their role in disease development, and potential treatment implications.
Keywords: hematological malignancies; lipid peroxidation; oxidative stress; protein carbonylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Battisti V., Maders L.D.K., Bagatini M.D., Santos K.F., Spanevello R.M., Maldonado P.A., Brulé A.O., do Carmo Araújo M., Schetinger M.R.C., Morsch V.M. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin. Biochem. 2008;41:511–518. doi: 10.1016/j.clinbiochem.2008.01.027. - DOI - PubMed
-
- Ahmad R., Tripathi A.K., Tripathi P., Singh S., Singh R., Singh R.K. Malondialdehyde and protein carbonyl as biomarkers for oxidative stress and disease progression in patients with chronic myeloid leukemia. In Vivo. 2008;22:525–528. - PubMed
-
- Ahmad R., Tripathi A.K., Tripathi P., Singh R., Singh S., Singh R.K. Studies on lipid peroxidation and non-enzymatic antioxidant status as indices of oxidative stress in patients with chronic myeloid leukaemia. Singap. Med. J. 2010;51:110–115. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

