Alterations of Glycosphingolipid Glycans and Chondrogenic Markers during Differentiation of Human Induced Pluripotent Stem Cells into Chondrocytes
- PMID: 33271874
- PMCID: PMC7760376
- DOI: 10.3390/biom10121622
Alterations of Glycosphingolipid Glycans and Chondrogenic Markers during Differentiation of Human Induced Pluripotent Stem Cells into Chondrocytes
Abstract
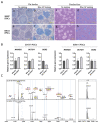
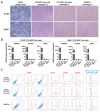
Due to the limited intrinsic healing potential of cartilage, injury to this tissue may lead to osteoarthritis. Human induced pluripotent stem cells (iPSCs), which can be differentiated into chondrocytes, are a promising source of cells for cartilage regenerative therapy. Currently, however, the methods for evaluating chondrogenic differentiation of iPSCs are very limited; the main techniques are based on the detection of chondrogenic genes and histological analysis of the extracellular matrix. The cell surface is coated with glycocalyx, a layer of glycoconjugates including glycosphingolipids (GSLs) and glycoproteins. The glycans in glycoconjugates play important roles in biological events, and their expression and structure vary widely depending on cell types and conditions. In this study, we performed a quantitative GSL-glycan analysis of human iPSCs, iPSC-derived mesenchymal stem cell like cells (iPS-MSC like cells), iPS-MSC-derived chondrocytes (iPS-MSC-CDs), bone marrow-derived mesenchymal stem cells (BMSCs), and BMSC-derived chondrocytes (BMSC-CDs) using glycoblotting technology. We found that GSL-glycan profiles differed among cell types, and that the GSL-glycome underwent a characteristic alteration during the process of chondrogenic differentiation. Furthermore, we analyzed the GSL-glycome of normal human cartilage and found that it was quite similar to that of iPS-MSC-CDs. This is the first study to evaluate GSL-glycan structures on human iPS-derived cartilaginous particles under micromass culture conditions and those of normal human cartilage. Our results indicate that GSL-glycome analysis is useful for evaluating target cell differentiation and can thus support safe regenerative medicine.
Keywords: aminolysis-SALSA; cartilage injury; chondrocytes; glycomics; glycosphingolipid; human induced pluripotent stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Evaluation of Residual Human-Induced Pluripotent Stem Cells in Human Chondrocytes by Cell Type-Specific Glycosphingolipid Glycome Analysis Based on the Aminolysis-SALSA Technique.Int J Mol Sci. 2019 Dec 28;21(1):231. doi: 10.3390/ijms21010231. Int J Mol Sci. 2019. PMID: 31905707 Free PMC article.
-
Footprint-free human induced pluripotent stem cells from articular cartilage with redifferentiation capacity: a first step toward a clinical-grade cell source.Stem Cells Transl Med. 2014 Apr;3(4):433-47. doi: 10.5966/sctm.2013-0138. Epub 2014 Mar 6. Stem Cells Transl Med. 2014. PMID: 24604283 Free PMC article.
-
Chondrogenic Progenitor Cells Exhibit Superiority Over Mesenchymal Stem Cells and Chondrocytes in Platelet-Rich Plasma Scaffold-Based Cartilage Regeneration.Am J Sports Med. 2019 Jul;47(9):2200-2215. doi: 10.1177/0363546519854219. Epub 2019 Jun 13. Am J Sports Med. 2019. PMID: 31194571
-
The Potency of Induced Pluripotent Stem Cells in Cartilage Regeneration and Osteoarthritis Treatment.Adv Exp Med Biol. 2018;1079:55-68. doi: 10.1007/5584_2017_141. Adv Exp Med Biol. 2018. PMID: 29270885 Review.
-
Mechanotransducive Biomimetic Systems for Chondrogenic Differentiation In Vitro.Int J Mol Sci. 2021 Sep 7;22(18):9690. doi: 10.3390/ijms22189690. Int J Mol Sci. 2021. PMID: 34575847 Free PMC article. Review.
Cited by
-
Hedgehog Signalling Contributes to Trauma-Induced Tendon Heterotopic Ossification and Regulates Osteogenesis through Antioxidant Pathway in Tendon-Derived Stem Cells.Antioxidants (Basel). 2022 Nov 16;11(11):2265. doi: 10.3390/antiox11112265. Antioxidants (Basel). 2022. PMID: 36421451 Free PMC article.
-
Ceiling culture of human mature white adipocytes with a browning agent: A novel approach to induce transdifferentiation into beige adipocytes.Front Bioeng Biotechnol. 2022 Aug 15;10:905194. doi: 10.3389/fbioe.2022.905194. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36046675 Free PMC article.
-
The positive impact of the NtTAS14-like1 gene on osmotic stress response in Nicotiana tabacum.Plant Cell Rep. 2023 Dec 29;43(1):25. doi: 10.1007/s00299-023-03118-2. Plant Cell Rep. 2023. PMID: 38155260
-
Glycosphingolipids in Osteoarthritis and Cartilage-Regeneration Therapy: Mechanisms and Therapeutic Prospects Based on a Narrative Review of the Literature.Int J Mol Sci. 2024 Apr 30;25(9):4890. doi: 10.3390/ijms25094890. Int J Mol Sci. 2024. PMID: 38732111 Free PMC article. Review.
-
Gangliosides and Their Role in Multilineage Differentiation of Mesenchymal Stem Cells.Biomedicines. 2022 Dec 2;10(12):3112. doi: 10.3390/biomedicines10123112. Biomedicines. 2022. PMID: 36551867 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

