Versatile Types of Polysaccharide-Based Drug Delivery Systems: From Strategic Design to Cancer Therapy
- PMID: 33271967
- PMCID: PMC7729619
- DOI: 10.3390/ijms21239159
Versatile Types of Polysaccharide-Based Drug Delivery Systems: From Strategic Design to Cancer Therapy
Abstract
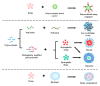
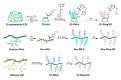
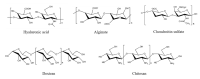
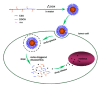
Chemotherapy is still the most direct and effective means of cancer therapy nowadays. The proposal of drug delivery systems (DDSs) has effectively improved many shortcomings of traditional chemotherapy drugs. The technical support of DDSs lies in their excellent material properties. Polysaccharides include a series of natural polymers, such as chitosan, hyaluronic acid, and alginic acid. These polysaccharides have good biocompatibility and degradability, and they are easily chemical modified. Therefore, polysaccharides are ideal candidate materials to construct DDSs, and their clinical application prospects have been favored by researchers. On the basis of versatile types of polysaccharides, this review elaborates their applications from strategic design to cancer therapy. The construction and modification methods of polysaccharide-based DDSs are specifically explained, and the latest research progress of polysaccharide-based DDSs in cancer therapy are also summarized. The purpose of this review is to provide a reference for the design and preparation of polysaccharide-based DDSs with excellent performance.
Keywords: cancer therapy; chemotherapy; drug delivery system (DDS); polysaccharide.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures






References
-
- Chen Z., Zhao K., Bu W., Ni D., Shi J. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. Int. Ed. Engl. 2014;54:1770–1774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

