Comparison of two ASC-derived therapeutics in an in vitro OA model: secretome versus extracellular vesicles
- PMID: 33272318
- PMCID: PMC7711257
- DOI: 10.1186/s13287-020-02035-5
Comparison of two ASC-derived therapeutics in an in vitro OA model: secretome versus extracellular vesicles
Abstract
Background: In the last years, several clinical trials have proved the safety and efficacy of adipose-derived stem/stromal cells (ASC) in contrasting osteoarthritis (OA). Since ASC act mainly through paracrine mechanisms, their secretome (conditioned medium, CM) represents a promising therapeutic alternative. ASC-CM is a complex cocktail of proteins, nucleic acids, and lipids released as soluble factors and/or conveyed into extracellular vesicles (EV). Here, we investigate its therapeutic potential in an in vitro model of OA.
Methods: Human articular chondrocytes (CH) were induced towards an OA phenotype by 10 ng/ml TNFα in the presence of either ASC-CM or EV, both deriving from 5 × 105 cells, to evaluate the effect on hypertrophic, catabolic, and inflammatory markers.
Results: Given the same number of donor cells, our data reveal a higher therapeutic potential of ASC-CM compared to EV alone that was confirmed by its enrichment in chondroprotective factors among which TIMP-1 and -2 stand out. In details, only ASC-CM significantly decreased MMP activity (22% and 29% after 3 and 6 days) and PGE2 expression (up to 40% at day 6) boosted by the inflammatory cytokine. Conversely, both treatments down-modulated of ~ 30% the hypertrophic marker COL10A1.
Conclusions: These biological and molecular evidences of ASC-CM beneficial action on CH with an induced OA phenotype may lay the basis for its future clinical translation as a cell-free therapeutic in the management of OA.
Keywords: Adipose-derived stem/stromal cells; Chondrocytes; Extracellular vesicles; Hypertrophy; MMP; Osteoarthritis; PGE2; Secretome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
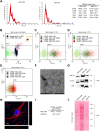
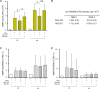
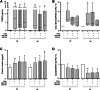

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

