Durability of Triple Combination Therapy Versus Stepwise Addition Therapy in Patients With New-Onset T2DM: 3-Year Follow-up of EDICT
- PMID: 33273042
- PMCID: PMC7818318
- DOI: 10.2337/dc20-0978
Durability of Triple Combination Therapy Versus Stepwise Addition Therapy in Patients With New-Onset T2DM: 3-Year Follow-up of EDICT
Abstract
Objective: To compare the long-term efficacy of initiating therapy with metformin/pioglitazone/exenatide in patients with new-onset type 2 diabetes mellitus (T2DM) versus sequential addition of metformin followed by glipizide and insulin.
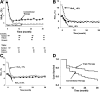
Research design and methods: Drug-naive patients (N = 318) with new-onset T2DM were randomly assigned to receive for 3 years either 1) combination therapy with metformin, pioglitazone, and exenatide (triple therapy) or 2) sequential addition of metformin followed by glipizide and insulin (conventional therapy) to maintain HbA1c at <6.5% (48 mmol/mol). Insulin sensitivity and β-cell function were measured at baseline and 3 years. The primary outcome was the difference in HbA1c between the groups at 3 years.
Results: Baseline HbA1c ± SEM values were 9.0% ± 0.2% and 8.9% ± 0.2% in the triple therapy and conventional therapy groups, respectively. The decrease in HbA1c resulting from triple therapy was greater at 6 months than that produced by conventional therapy (0.30% [95% CI 0.21-0.39]; P = 0.001), and the HbA1c reduction was maintained at 3 years in patients receiving triple therapy compared with conventional therapy (6.4% ± 0.1% and 6.9% ± 0.1%, respectively), despite intensification of antihyperglycemic therapy in the latter. Thus, the difference in HbA1c between the two treatment groups at 3 years was 0.50% (95% CI 0.39-0.61; P < 0.0001). Triple therapy produced a threefold increase in insulin sensitivity and 30-fold increase in β-cell function. In conventional therapy, insulin sensitivity did not change and β-cell function increased by only 34% (both P < 0.0001 vs. triple therapy).
Conclusions: Triple therapy with agents that improve insulin sensitivity and β-cell function in patients with new-onset T2DM produces greater, more durable HbA1c reduction than agents that lower glucose levels without correcting the underlying metabolic defects.
Trial registration: ClinicalTrials.gov NCT01107717.
© 2020 by the American Diabetes Association.
Figures


References
-
- Gulli G, Ferrannini E, Stern M, Haffner S, DeFronzo RA. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes 1992;41:1575–1586 - PubMed
-
- Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 1990;113:909–915 - PubMed
-
- DeFronzo RA Lilly lecture 1987. The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667–687 - PubMed
-
- Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA; San Antonio metabolism study . Beta-cell dysfunction and glucose intolerance: results from the San Antonio Metabolism (SAM) study. Diabetologia 2004;47:31–39 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

