Hierarchical routing in carbon metabolism favors iron-scavenging strategy in iron-deficient soil Pseudomonas species
- PMID: 33273114
- PMCID: PMC7768705
- DOI: 10.1073/pnas.2016380117
Hierarchical routing in carbon metabolism favors iron-scavenging strategy in iron-deficient soil Pseudomonas species
Abstract
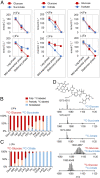
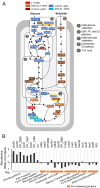
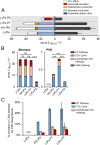
High-affinity iron (Fe) scavenging compounds, or siderophores, are widely employed by soil bacteria to survive scarcity in bioavailable Fe. Siderophore biosynthesis relies on cellular carbon metabolism, despite reported decrease in both carbon uptake and Fe-containing metabolic proteins in Fe-deficient cells. Given this paradox, the metabolic network required to sustain the Fe-scavenging strategy is poorly understood. Here, through multiple 13C-metabolomics experiments with Fe-replete and Fe-limited cells, we uncover how soil Pseudomonas species reprogram their metabolic pathways to prioritize siderophore biosynthesis. Across the three species investigated (Pseudomonas putida KT2440, Pseudomonas protegens Pf-5, and Pseudomonas putida S12), siderophore secretion is higher during growth on gluconeogenic substrates than during growth on glycolytic substrates. In response to Fe limitation, we capture decreased flux toward the tricarboxylic acid (TCA) cycle during the metabolism of glycolytic substrates but, due to carbon recycling to the TCA cycle via enhanced anaplerosis, the metabolism of gluconeogenic substrates results in an increase in both siderophore secretion (up to threefold) and Fe extraction (up to sixfold) from soil minerals. During simultaneous feeding on the different substrate types, Fe deficiency triggers a hierarchy in substrate utilization, which is facilitated by changes in protein abundances for substrate uptake and initial catabolism. Rerouted metabolism further promotes favorable fluxes in the TCA cycle and the gluconeogenesis-anaplerosis nodes, despite decrease in several proteins in these pathways, to meet carbon and energy demands for siderophore precursors in accordance with increased proteins for siderophore biosynthesis. Hierarchical carbon metabolism thus serves as a critical survival strategy during the metal nutrient deficiency.
Keywords: Pseudomonas putida; bacteria; iron limitation; metabolomics; siderophore.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures





Similar articles
-
Analogous Metabolic Decoupling in Pseudomonas putida and Comamonas testosteroni Implies Energetic Bypass to Facilitate Gluconeogenic Growth.mBio. 2021 Dec 21;12(6):e0325921. doi: 10.1128/mbio.03259-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903058 Free PMC article.
-
Bypasses in intracellular glucose metabolism in iron-limited Pseudomonas putida.Microbiologyopen. 2016 Feb;5(1):3-20. doi: 10.1002/mbo3.287. Epub 2015 Sep 16. Microbiologyopen. 2016. PMID: 26377487 Free PMC article.
-
Comparative Genomics, Siderophore Production, and Iron Scavenging Potential of Root Zone Soil Bacteria Isolated from 'Concord' Grape Vineyards.Microb Ecol. 2019 Oct;78(3):699-713. doi: 10.1007/s00248-019-01324-8. Epub 2019 Feb 15. Microb Ecol. 2019. PMID: 30770943
-
Gene regulation of siderophore-mediated iron acquisition in Pseudomonas: not only the Fur repressor.Mol Microbiol. 1995 Aug;17(4):603-10. doi: 10.1111/j.1365-2958.1995.mmi_17040603.x. Mol Microbiol. 1995. PMID: 8801415 Review.
-
Iron uptake and metabolism in pseudomonads.Appl Microbiol Biotechnol. 2010 May;86(6):1637-45. doi: 10.1007/s00253-010-2550-2. Epub 2010 Mar 30. Appl Microbiol Biotechnol. 2010. PMID: 20352420 Review.
Cited by
-
Nutrient dominance governs the assembly of microbial communities in mixed nutrient environments.Elife. 2021 Apr 20;10:e65948. doi: 10.7554/eLife.65948. Elife. 2021. PMID: 33877964 Free PMC article.
-
Perspective on the biotechnological production of bacterial siderophores and their use.Appl Microbiol Biotechnol. 2022 Jun;106(11):3985-4004. doi: 10.1007/s00253-022-11995-y. Epub 2022 Jun 8. Appl Microbiol Biotechnol. 2022. PMID: 35672469 Review.
-
Effects of Phosphonate Herbicides on the Secretions of Plant-Beneficial Compounds by Two Plant Growth-Promoting Soil Bacteria: A Metabolomics Investigation.ACS Environ Au. 2021 Nov 18;2(2):136-149. doi: 10.1021/acsenvironau.1c00030. eCollection 2022 Mar 16. ACS Environ Au. 2021. PMID: 37101584 Free PMC article.
-
Characterization of the antagonistic potential of the glyphosate-tolerant Pseudomonas resinovorans SZMC 25872 strain against the plant pathogenic bacterium Agrobacterium tumefaciens.Front Plant Sci. 2022 Nov 28;13:1034237. doi: 10.3389/fpls.2022.1034237. eCollection 2022. Front Plant Sci. 2022. PMID: 36518497 Free PMC article.
-
Analogous Metabolic Decoupling in Pseudomonas putida and Comamonas testosteroni Implies Energetic Bypass to Facilitate Gluconeogenic Growth.mBio. 2021 Dec 21;12(6):e0325921. doi: 10.1128/mbio.03259-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903058 Free PMC article.
References
-
- Boukhalfa H., Crumbliss A. L., Chemical aspects of siderophore mediated iron transport. Biometals 15, 325–339 (2002). - PubMed
-
- Braun V., Hantke K., Recent insights into iron import by bacteria. Curr. Opin. Chem. Biol. 15, 328–334 (2011). - PubMed
-
- Konhauser K. O., Kappler A., Roden E. E., Iron in microbial metabolisms. Elements 7, 89–93 (2011).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

