In vivo O2 imaging in hepatic tissues by phosphorescence lifetime imaging microscopy using Ir(III) complexes as intracellular probes
- PMID: 33273499
- PMCID: PMC7713648
- DOI: 10.1038/s41598-020-76878-6
In vivo O2 imaging in hepatic tissues by phosphorescence lifetime imaging microscopy using Ir(III) complexes as intracellular probes
Abstract
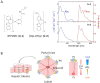
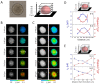
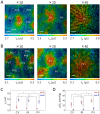
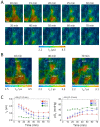
Phosphorescence lifetime imaging microscopy (PLIM) combined with an oxygen (O2)-sensitive luminescent probe allows for high-resolution O2 imaging of living tissues. Herein, we present phosphorescent Ir(III) complexes, (btp)2Ir(acac-DM) (Ir-1) and (btp-OH)3Ir (Ir-2), as useful O2 probes for PLIM measurement. These small-molecule probes were efficiently taken up into cultured cells and accumulated in specific organelles. Their excellent cell-permeable properties allowed for efficient staining of three-dimensional cell spheroids, and thereby phosphorescence lifetime measurements enabled the evaluation of the O2 level and distribution in spheroids, including the detection of alterations in O2 levels by metabolic stimulation with an effector. We took PLIM images of hepatic tissues of living mice by intravenously administrating these probes. The PLIM images clearly visualized the O2 gradient in hepatic lobules with cellular-level resolution, and the O2 levels were derived based on calibration using cultured cells; the phosphorescence lifetime of Ir-1 gave reasonable O2 levels, whereas Ir-2 exhibited much lower O2 levels. Intravenous administration of NH4Cl to mice caused the hepatic tissues to experience hypoxia, presumably due to O2 consumption to produce ATP required for ammonia detoxification, suggesting that the metabolism of the probe molecule might affect liver O2 levels.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

