Infectious RNA vaccine protects mice against chikungunya virus infection
- PMID: 33273501
- PMCID: PMC7712826
- DOI: 10.1038/s41598-020-78009-7
Infectious RNA vaccine protects mice against chikungunya virus infection
Abstract
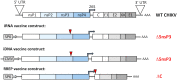
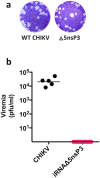
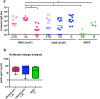
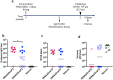
We describe a novel vaccine platform that can generate protective immunity to chikungunya virus (CHIKV) in C57BL/6J mice after a single immunization by employing an infectious RNA (iRNA), which upon introduction into a host cell launches an infectious attenuated virus. We and others have previously reported that an engineered deletion of 183 nucleotides in the nsP3 gene attenuates chikungunya virus (CHIKV) and reduces in vivo viral replication and viremia after challenge in mice, macaques and man. Here, we demonstrated that in vitro transfection of iRNA carrying the nsP3 deletion generated infectious viruses, and after intramuscular injection, the iRNA induced robust antibody responses in mice. The iRNA was superior at eliciting binding and neutralizing antibody responses as compared to a DNA vaccine encoding the same RNA (iDNA) or a non-propagating RNA replicon (RREP) lacking the capsid encoding gene. Subsequent challenge with a high dose of CHIKV demonstrated that the antibody responses induced by this vaccine candidate protected animals from viremia. The iRNA approach constitutes a novel vaccine platform with the potential to impact the spread of CHIKV. Moreover, we believe that this approach is likely applicable also to other positive-strand viruses.
Conflict of interest statement
The authors declare no conflict of interest. KL is currently employed by Eurocine-Vaccines, but the work occurred at his previous organization (Karolinska Institutet). No conflict of interests exists.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

