Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells
- PMID: 33273741
- PMCID: PMC9042317
- DOI: 10.1038/s41587-020-00763-w
Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells
Abstract
Cortico-striatal projections are critical components of forebrain circuitry that regulate motivated behaviors. To enable the study of the human cortico-striatal pathway and how its dysfunction leads to neuropsychiatric disease, we developed a method to convert human pluripotent stem cells into region-specific brain organoids that resemble the developing human striatum and include electrically active medium spiny neurons. We then assembled these organoids with cerebral cortical organoids in three-dimensional cultures to form cortico-striatal assembloids. Using viral tracing and functional assays in intact or sliced assembloids, we show that cortical neurons send axonal projections into striatal organoids and form synaptic connections. Medium spiny neurons mature electrophysiologically following assembly and display calcium activity after optogenetic stimulation of cortical neurons. Moreover, we derive cortico-striatal assembloids from patients with a neurodevelopmental disorder caused by a deletion on chromosome 22q13.3 and capture disease-associated defects in calcium activity, showing that this approach will allow investigation of the development and functional assembly of cortico-striatal connectivity using patient-derived cells.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
Stanford University has filed a provisional patent application covering the protocol and methods for the generation of striatal spheroids and cortico-striatal assembloids. M.H.P. is a consultant for and has equity interest in CRIPR Tx. During the duration of this study, K.I. was an employee of Daiichi-Sankyo Co., Ltd, although the company had no input on the design, execution, interpretation, or publication of the data.
Figures



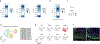


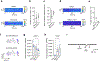








Comment in
-
Assembling organs in vitro.Nat Methods. 2021 Feb;18(2):119. doi: 10.1038/s41592-021-01068-9. Nat Methods. 2021. PMID: 33542506 No abstract available.
References
-
- Alexander GE, DeLong MR & Strick PL Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9, 357–381 (1986). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

