Current understanding of glucose transporter 4 expression and functional mechanisms
- PMID: 33274014
- PMCID: PMC7672939
- DOI: 10.4331/wjbc.v11.i3.76
Current understanding of glucose transporter 4 expression and functional mechanisms
Abstract
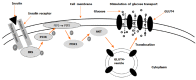
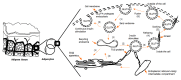
Glucose is used aerobically and anaerobically to generate energy for cells. Glucose transporters (GLUTs) are transmembrane proteins that transport glucose across the cell membrane. Insulin promotes glucose utilization in part through promoting glucose entry into the skeletal and adipose tissues. This has been thought to be achieved through insulin-induced GLUT4 translocation from intracellular compartments to the cell membrane, which increases the overall rate of glucose flux into a cell. The insulin-induced GLUT4 translocation has been investigated extensively. Recently, significant progress has been made in our understanding of GLUT4 expression and translocation. Here, we summarized the methods and reagents used to determine the expression levels of Slc2a4 mRNA and GLUT4 protein, and GLUT4 translocation in the skeletal muscle, adipose tissues, heart and brain. Overall, a variety of methods such real-time polymerase chain reaction, immunohistochemistry, fluorescence microscopy, fusion proteins, stable cell line and transgenic animals have been used to answer particular questions related to GLUT4 system and insulin action. It seems that insulin-induced GLUT4 translocation can be observed in the heart and brain in addition to the skeletal muscle and adipocytes. Hormones other than insulin can induce GLUT4 translocation. Clearly, more studies of GLUT4 are warranted in the future to advance of our understanding of glucose homeostasis.
Keywords: Adipocytes; Antibodies; Brain; Glucose transporter 4; Heart; Insulin; Skeletal muscle.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All authors declare that there is no conflict of interest to report.
Figures
References
-
- La Flamme KE, Mor G, Gong D, La Tempa T, Fusaro VA, Grimes CA, Desai TA. Nanoporous alumina capsules for cellular macroencapsulation: transport and biocompatibility. Diabetes Technol Ther. 2005;7:684–694. - PubMed
-
- Stipanuk MH, Caudill MA. Biochemical, Physiological, and Molecular Aspects of Human Nutrition. 3rd ed. St. Louis, MO: Elsevier, 2012: 968.
-
- Quistgaard EM, Löw C, Guettou F, Nordlund P. Understanding transport by the major facilitator superfamily (MFS): structures pave the way. Nat Rev Mol Cell Biol. 2016;17:123–132. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

