Unconventional Macrocyclizations in Natural Product Synthesis
- PMID: 33274267
- PMCID: PMC7706100
- DOI: 10.1021/acscentsci.0c00599
Unconventional Macrocyclizations in Natural Product Synthesis
Abstract
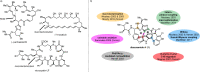
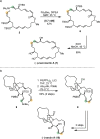
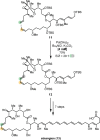
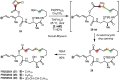
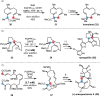
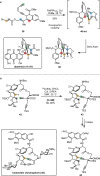
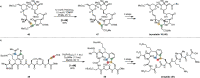
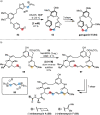
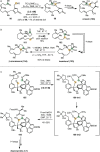
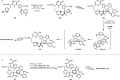
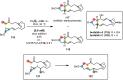
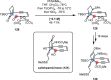
Over the past several decades, macrocyclic compounds have emerged as increasingly significant therapeutic candidates in drug discovery. Their pharmacological activity hinges on their rotationally restricted three-dimensional orientation, resulting in a unique conformational preorganization and a high enthalpic gain as a consequence of high-affinity macrocycle-protein binding interactions. Synthetic access to macrocyclic drug candidates is therefore crucial. From a synthetic point of view, the efficiency of macrocyclization events commonly suffers from entropic penalties as well as undesired intermolecular couplings (oligomerization). Although over the past several decades ring-closing metathesis, macrolactonization, or macrolactamization have become strategies of choice, the toolbox of organic synthesis provides a great number of versatile transformations beyond the aforementioned. This Outlook focuses on a selection of examples employing what we term unconventional macrocyclizations toward the synthesis of natural products or analogues.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



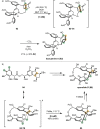

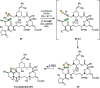
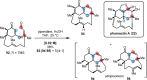
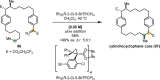



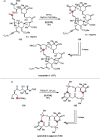
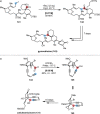
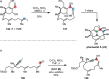
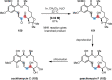
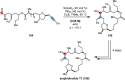
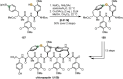

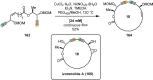
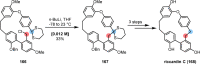
References
-
- Newman D. J.; Cragg G. M.. Bioactive Macrocycles from Nature. In Macrocycles in Drug Discovery; Royal Society of Chemistry, 2015; pp 1–36.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

