Ethanol induces persistent potentiation of 5-HT3 receptor-stimulated GABA release at synapses on rat hippocampal CA1 neurons
- PMID: 33275959
- PMCID: PMC11009934
- DOI: 10.1016/j.neuropharm.2020.108415
Ethanol induces persistent potentiation of 5-HT3 receptor-stimulated GABA release at synapses on rat hippocampal CA1 neurons
Abstract
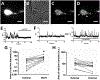
Several studies have shown that ethanol (EtOH) can enhance the activity of GABAergic synapses via presynaptic mechanisms, including in hippocampal CA1 neurons. The serotonin type 3 receptor (5-HT3-R) has been implicated in the neural actions of ethanol (EtOH) and in modulation of GABA release from presynaptic terminals. In the present study, we investigated EtOH modulation of GABA release induced by 5-HT3-R activation using the mechanically isolated neuron/bouton preparation from the rat CA1 hippocampal subregion. EtOH application before and during exposure to the selective 5-HT3 receptor agonist, m-chlorophenylbiguanide (mCPBG) potentiated the mCPBG-induced increases in the peak frequency and charge transfer of spontaneous GABAergic inhibitory postsynaptic currents. Interestingly, the potentiation was maintained even after EtOH was removed from the preparation. A protein kinase A inhibitor reduced the magnitude of EtOH potentiation. Fluorescent Ca2+ imaging showed that Ca2+ transients in the presynaptic terminals increased during EtOH exposure. These findings indicate that EtOH produces long-lasting potentiation of 5-HT3-induced GABA release by modulating calcium levels, via a process involving cAMP-mediated signaling in presynaptic terminals.
Keywords: Alcohol; GABAergic synapse; Hippocampus; Neuron/bouton; Serotonin.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interestCOI
The authors declare no competing financial interests.
Figures






References
-
- Akaike N, Moorhouse AJ, 2003. Techniques: applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol. Sci 24, 44–47. - PubMed
-
- Akaike N, Murakami N, Katsurabayashi S, Jin YH, Imazawa T, 2002. Focal stimulation of single GABAergic presynaptic boutons on the rat hippocampal neuron. Neurosci. Res 42, 187–195. - PubMed
-
- Asher O, Cunningham TD, Yao L, Gordon AS, Diamond I, 2002. Ethanol stimulates cAMP-responsive element (CRE)-mediated transcription via CRE-binding protein and cAMP-dependent protein kinase. J. Pharmacol. Exp. Therapeut 301, 66–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

