CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma
- PMID: 33276030
- PMCID: PMC9662232
- DOI: 10.1016/j.jhep.2020.11.037
CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma
Abstract
Background & aims: While cholangiocarcinomas (CCAs) commonly express programmed cell death 1 (PD-1) and its ligand (PD-L1), they respond poorly to immune checkpoint inhibitors (ICIs). We aimed to determine whether stimulating antigen-presenting cells, including macrophages and dendritic cells, using a CD40 agonist could improve this response.
Methods: We compared treatment responses in subcutaneous, orthotopic, and 2 plasmid-based murine intrahepatic CCA (iCCA) models. Mice were treated for 4 weeks with weekly IgG control, a CD40 agonistic antibody, anti-PD-1, or the combination of both (anti-CD40/PD-1). Flow cytometric (FACS) analysis of lymphocytes and myeloid cell populations (including activation status) was performed. We used dendritic cell knockout mice, and macrophage, CD4+ and CD8+ T cell depletion models to identify effector cells. Anti-CD40/PD-1 was combined with chemotherapy (gemcitabine/cisplatin) to test for improved therapeutic efficacy.
Results: In all 4 models, anti-PD-1 alone was minimally efficacious. Mice exhibited a moderate response to CD40 agonist monotherapy. Combination anti-CD40/PD-1 therapy led to a significantly greater reduction in tumor burden. FACS demonstrated increased number and activation of CD4+ and CD8+ T cells, natural killer cells, and myeloid cells in tumor and non-tumor liver tissue of tumor-bearing mice treated with anti-CD40/PD-1. Depletion of macrophages, dendritic cells, CD4+ T cells, or CD8+ T cells abrogated treatment efficacy. Combining anti-CD40/PD-1 with gemcitabine/cisplatin resulted in a significant survival benefit compared to gemcitabine/cisplatin alone.
Conclusion: CD40-mediated activation of macrophages and dendritic cells in iCCA significantly enhances response to anti-PD-1 therapy. This regimen may enhance the efficacy of first-line chemotherapy.
Lay summary: Checkpoint inhibition, a common form of immune therapy, is generally ineffective for the treatment of cholangiocarcinoma. These tumors suppress the infiltration and function of surrounding immune cells. Stimulating immune cells such as macrophages and dendritic cells via the CD40 receptor activates downstream immune cells and enhances the response to checkpoint inhibitors.
Keywords: Antigen-presenting cell; CD40 agonist; Cholangiocarcinoma; Dendritic cell; Immune checkpoint; Immunotherapy; Liver cancer; Macrophage; NK cell; T cell; Tumor-infiltrating lymphocyte.
Published by Elsevier B.V.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

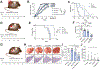

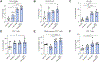
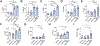
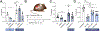
Comment in
-
CD40-agonist: A new avenue for immunotherapy combinations in cholangiocarcinoma.J Hepatol. 2021 May;74(5):1021-1024. doi: 10.1016/j.jhep.2021.01.030. Epub 2021 Feb 19. J Hepatol. 2021. PMID: 33612309 No abstract available.
References
-
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. The New Engl J Med 2010;362:1273–1281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

