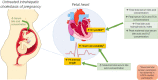
Fetal cardiac dysfunction in intrahepatic cholestasis of pregnancy is associated with elevated serum bile acid concentrations
- PMID: 33276032
- PMCID: PMC8062912
- DOI: 10.1016/j.jhep.2020.11.038
Fetal cardiac dysfunction in intrahepatic cholestasis of pregnancy is associated with elevated serum bile acid concentrations
Abstract
Background & aims: Intrahepatic cholestasis of pregnancy (ICP) is associated with an increased risk of stillbirth. This study aimed to assess the relationship between bile acid concentrations and fetal cardiac dysfunction in patients with ICP who were or were not treated with ursodeoxycholic acid (UDCA).
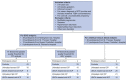
Methods: Bile acid profiles and NT-proBNP, a marker of ventricular dysfunction, were assayed in umbilical venous serum from 15 controls and 76 ICP cases (36 untreated, 40 UDCA-treated). Fetal electrocardiogram traces were obtained from 43 controls and 48 ICP cases (26 untreated, 22 UDCA-treated). PR interval length and heart rate variability (HRV) parameters were measured in 2 behavioral states (quiet and active sleep).
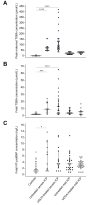
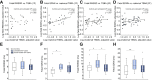
Results: In untreated ICP, fetal total serum bile acid (TSBA) concentrations (r = 0.49, p = 0.019), hydrophobicity index (r = 0.20, p = 0.039), glycocholate concentrations (r = 0.56, p = 0.007) and taurocholate concentrations (r = 0.44, p = 0.039) positively correlated with fetal NT-proBNP. Maternal TSBA (r = 0.40, p = 0.026) and alanine aminotransferase (r = 0.40, p = 0.046) also positively correlated with fetal NT-proBNP. There were no significant correlations between maternal or fetal serum bile acid concentrations and fetal HRV parameters or NT-proBNP concentrations in the UDCA-treated cohort. Fetal PR interval length positively correlated with maternal TSBA in untreated (r = 0.46, p = 0.027) and UDCA-treated ICP (r = 0.54, p = 0.026). Measures of HRV in active sleep and quiet sleep were significantly higher in untreated ICP cases than controls. HRV values in UDCA-treated cases did not differ from controls.
Conclusions: Elevated fetal and maternal serum bile acid concentrations in untreated ICP are associated with an abnormal fetal cardiac phenotype characterized by increased NT-proBNP concentration, PR interval length and HRV. UDCA treatment partially attenuates this phenotype.
Lay summary: The risk of stillbirth in intrahepatic cholestasis of pregnancy (ICP) is linked to the level of bile acids in the mother which are thought to disrupt the baby's heart rhythm. We found that babies of women with untreated ICP have abnormally functioning hearts compared to those without ICP, and the degree of abnormality is closely linked to the level of harmful bile acids in the mother and baby's blood. Babies of women with ICP who received treatment with the drug UDCA do not have the same level of abnormality in their hearts, suggesting that UDCA could be a beneficial treatment in some ICP cases, although further clinical trials are needed to confirm this.
Keywords: Bile; Cholic acid; Female; Heart rate; Intrahepatic cholestasis of pregnancy; Pregnancy; Stillbirth; Ursodeoxycholic acid; Ventricular dysfunction.
Copyright © 2021 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest BHG has previously served as a director for Monica Healthcare Limited and has no commercial or financial connections in the company. CW and HUM are consultants with Mirum Pharmaceuticals and CW is a consultant for GlaxoSmithKline. The remaining authors have no conflicts of interest to disclose. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

