Roadmap for the multiscale coupling of biochemical and mechanical signals during development
- PMID: 33276350
- PMCID: PMC8380410
- DOI: 10.1088/1478-3975/abd0db
Roadmap for the multiscale coupling of biochemical and mechanical signals during development
Abstract
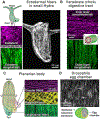
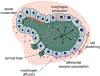
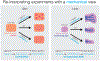
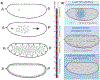
The way in which interactions between mechanics and biochemistry lead to the emergence of complex cell and tissue organization is an old question that has recently attracted renewed interest from biologists, physicists, mathematicians and computer scientists. Rapid advances in optical physics, microscopy and computational image analysis have greatly enhanced our ability to observe and quantify spatiotemporal patterns of signalling, force generation, deformation, and flow in living cells and tissues. Powerful new tools for genetic, biophysical and optogenetic manipulation are allowing us to perturb the underlying machinery that generates these patterns in increasingly sophisticated ways. Rapid advances in theory and computing have made it possible to construct predictive models that describe how cell and tissue organization and dynamics emerge from the local coupling of biochemistry and mechanics. Together, these advances have opened up a wealth of new opportunities to explore how mechanochemical patterning shapes organismal development. In this roadmap, we present a series of forward-looking case studies on mechanochemical patterning in development, written by scientists working at the interface between the physical and biological sciences, and covering a wide range of spatial and temporal scales, organisms, and modes of development. Together, these contributions highlight the many ways in which the dynamic coupling of mechanics and biochemistry shapes biological dynamics: from mechanoenzymes that sense force to tune their activity and motor output, to collectives of cells in tissues that flow and redistribute biochemical signals during development.
Keywords: embryogenesis; morphogenesis; signalling.
Creative Commons Attribution license.
Figures











References
-
- Heemskerk I. Full of potential: pluripotent stem cells for the systems biology of embryonic patterning. Dev. Biol. 2019;460:86. - PubMed
-
- Haremaki T, Metzger JJ, Rito T, Ozair MZ, Etoc F and Brivanlou AH 2019. Self-organizing neuruloids model developmental aspects of Huntington’s disease in the ectodermal compartment Nat. Biotechnol 37 1198–208 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
