Correlates of protection against SARS-CoV-2 in rhesus macaques
- PMID: 33276369
- PMCID: PMC7906955
- DOI: 10.1038/s41586-020-03041-6
Correlates of protection against SARS-CoV-2 in rhesus macaques
Abstract
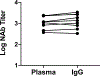
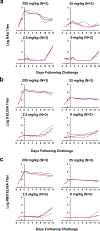
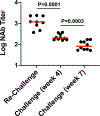
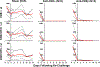
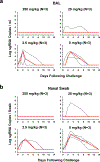
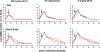
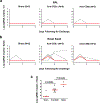
Recent studies have reported the protective efficacy of both natural1 and vaccine-induced2-7 immunity against challenge with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in rhesus macaques. However, the importance of humoral and cellular immunity for protection against infection with SARS-CoV-2 remains to be determined. Here we show that the adoptive transfer of purified IgG from convalescent rhesus macaques (Macaca mulatta) protects naive recipient macaques against challenge with SARS-CoV-2 in a dose-dependent fashion. Depletion of CD8+ T cells in convalescent macaques partially abrogated the protective efficacy of natural immunity against rechallenge with SARS-CoV-2, which suggests a role for cellular immunity in the context of waning or subprotective antibody titres. These data demonstrate that relatively low antibody titres are sufficient for protection against SARS-CoV-2 in rhesus macaques, and that cellular immune responses may contribute to protection if antibody responses are suboptimal. We also show that higher antibody titres are required for treatment of SARS-CoV-2 infection in macaques. These findings have implications for the development of SARS-CoV-2 vaccines and immune-based therapeutic agents.
Conflict of interest statement
Competing Interests
The authors declare no financial conflicts of interest. D.H.B. is a co-inventor on provisional SARS-CoV-2 vaccine patents (62/969,008; 62/994,630).
Figures


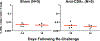


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

