Analysis of Gluten in Dried Yeast and Yeast-Containing Products
- PMID: 33276528
- PMCID: PMC7761069
- DOI: 10.3390/foods9121790
Analysis of Gluten in Dried Yeast and Yeast-Containing Products
Abstract
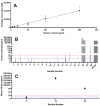
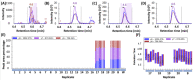
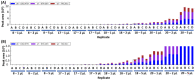
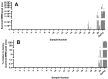
Yeast are commonly used in the preparation of foods and beverages such as beer and bread and may also be used on their own as a source of nutrients and flavoring. Because of the historical connection of yeast to products made from wheat and barley, consumers maintaining a gluten-free diet can have concerns about the safety of yeast ingredients. Analyzing the safety of yeast and yeast-containing products presents some difficulties, as the yeast organisms actively degrade any gluten in the product, raising questions on the appropriateness of detection by traditional antibody-based methods. This study examines a variety of yeast and yeast-containing products by competitive ELISA and liquid chromatography-mass spectrometry for the estimated level of gluten proteins. While samples such as yeast extracts and nutritional yeast contained gluten levels below the 20 mg/kg (or parts per million, ppm) threshold defined by Codex Alimentarius, one baking yeast and a nutritional yeast supplement sample contained higher levels of gluten. This study demonstrates that both competitive ELISA and liquid chromatography-mass spectrometry provide similar results in the detection of wheat and barley gluten in yeast-containing products.
Keywords: ELISA; LC–MS; gluten; yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bekatorou A., Psarianos C., Koutinas A.A. Production of Food Grade Yeasts. Food Technol. Biotechnol. 2006;44:407–415.
-
- Pasteur L. The Diseases of Beer, Their Causes, and the Means of Preventing Them. MacMillan & Co.; London, UK: 1879.
-
- Reed G., Nagodawithana T. Yeast Technology. 2nd ed. Van Nostrand Reinhold; New York, NY, USA: 1991. p. 381.
-
- Halasz A., Lasztity R. Use of Yeast Biomass in Food Production. CRC Press; Boca Raton, FL, USA: 1991.
-
- Chniti S., Djelal H., Hassouna M., Amrane A. Residue of dates from the food industry as a cheap new feedstock for ethanol production. Biomass Bioenergy. 2014;69:66–70. doi: 10.1016/j.biombioe.2014.07.011. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

