Synthetic Peptide ΔM4-Induced Cell Death Associated with Cytoplasmic Membrane Disruption, Mitochondrial Dysfunction and Cell Cycle Arrest in Human Melanoma Cells
- PMID: 33276536
- PMCID: PMC7730669
- DOI: 10.3390/molecules25235684
Synthetic Peptide ΔM4-Induced Cell Death Associated with Cytoplasmic Membrane Disruption, Mitochondrial Dysfunction and Cell Cycle Arrest in Human Melanoma Cells
Abstract
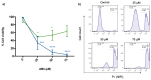
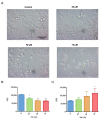
Melanoma is the most dangerous and lethal form of skin cancer, due to its ability to spread to different organs if it is not treated at an early stage. Conventional chemotherapeutics are failing as a result of drug resistance and weak tumor selectivity. Therefore, efforts to evaluate novel molecules for the treatment of skin cancer are necessary. Antimicrobial peptides have become attractive anticancer agents because they execute their biological activity with features such as a high potency of action, a wide range of targets, and high target specificity and selectivity. In the present study, the antiproliferative activity of the synthetic peptide ΔM4 on A375 human melanoma cells and spontaneously immortalized HaCaT human keratinocytes was investigated. The cytotoxic effect of ΔM4 treatment was evaluated through propidium iodide uptake by flow cytometry. The results indicated selective toxicity in A375 cells and, in order to further investigate the mode of action, assays were carried out to evaluate morphological changes, mitochondrial function, and cell cycle progression. The findings indicated that ΔM4 exerts its antitumoral effects by multitarget action, causing cell membrane disruption, a change in the mitochondrial transmembrane potential, an increase of reactive oxygen species, and cell cycle accumulation in S-phase. Further exploration of the peptide may be helpful in the design of novel anticancer peptides.
Keywords: antimicrobial peptides; antiproliferative peptides; cell cycle arrest; melanoma skin cancer; membrane integrity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cooper G.M., Hausman R.E. The Cell: A Molecular Approach. 4th ed. Sinauer Associates; Sunderland, MA, USA: 2007. The Development and Causes of Cancer; pp. 719–723.
-
- Steward B., Wild C.P. World Cancer Report 2014. WHO Press; Geneva, Switzerland: 2014. pp. 16–69.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

