Evidence-Based Use of Levothyroxine/Liothyronine Combinations in Treating Hypothyroidism: A Consensus Document
- PMID: 33276704
- PMCID: PMC8035928
- DOI: 10.1089/thy.2020.0720
Evidence-Based Use of Levothyroxine/Liothyronine Combinations in Treating Hypothyroidism: A Consensus Document
Abstract
Background: Fourteen clinical trials have not shown a consistent benefit of combination therapy with levothyroxine (LT4) and liothyronine (LT3). Despite the publication of these trials, combination therapy is widely used and patients reporting benefit continue to generate patient and physician interest in this area. Recent scientific developments may provide insight into this inconsistency and guide future studies. Methods: The American Thyroid Association (ATA), British Thyroid Association (BTA), and European Thyroid Association (ETA) held a joint conference on November 3, 2019 (live-streamed between Chicago and London) to review new basic science and clinical evidence regarding combination therapy with presentations and input from 12 content experts. After the presentations, the material was synthesized and used to develop Summary Statements of the current state of knowledge. After review and revision of the material and Summary Statements, there was agreement that there was equipoise for a new clinical trial of combination therapy. Consensus Statements encapsulating the implications of the material discussed with respect to the design of future clinical trials of LT4/LT3 combination therapy were generated. Authors voted upon the Consensus Statements. Iterative changes were made in several rounds of voting and after comments from ATA/BTA/ETA members. Results: Of 34 Consensus Statements available for voting, 28 received at least 75% agreement, with 13 receiving 100% agreement. Those with 100% agreement included studies being powered to study the effect of deiodinase and thyroid hormone transporter polymorphisms on study outcomes, inclusion of patients dissatisfied with their current therapy and requiring at least 1.2 μg/kg of LT4 daily, use of twice daily LT3 or preferably a slow-release preparation if available, use of patient-reported outcomes as a primary outcome (measured by a tool with both relevant content validity and responsiveness) and patient preference as a secondary outcome, and utilization of a randomized placebo-controlled adequately powered double-blinded parallel design. The remaining statements are presented as potential additional considerations. Discussion: This article summarizes the areas discussed and presents Consensus Statements to guide development of future clinical trials of LT4/LT3 combination therapy. The results of such redesigned trials are expected to be of benefit to patients and of value to inform future thyroid hormone replacement clinical practice guidelines treatment recommendations.
Keywords: clinical trial; combination therapy; hypothyroidism; levothyroxine; liothyronine; patient-reported outcomes.
Conflict of interest statement
J.J., A.R.C., H.H., E.A.M., L.C.M., A.M.S., and C.M.D. have no conflicts to discuss. A.C.B. is a consultant for Synthonics, Inc., Allergan, Inc., and BLA Technology LLC; F.S.C. is a consultant for IBSA and Acella; and T.W. is a consultant for AbbVie, Allergan, Inc., and Developer of ThyPRO and ThyPRO-30.
E.F. is a coinvestigator in a combination therapy trial funded by the Dutch government with funds provided to participating institutions (principal investigator [PI] Marco Medici). B.N. is the PI of a trial of synthetic combination therapy versus thyroid extract funded by the Danish government with funds provided to the institution. T.W. also serves as a volunteer consultant and steering committee member specifically with ThyPRO expertise for combination therapy trials with PIs Medici (above), B.N. (above), and Steen Bonnema (trial funded by the Danish government). C.M.D. serves as a volunteer external advisory board member for the trial with PI Medici (above).
Figures
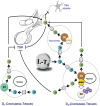
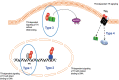
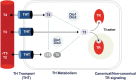
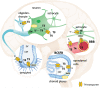

References
-
- Escobar-Morreale HF, del Rey FE, Obregón MJ, de Escobar GM. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. 1996;137(6):2490–2502 - PubMed
-
- Christoffolete MA, Ribeiro R, Singru P, Fekete C, da Silva WS, Gordon DF, Huang SA, Crescenzi A, Harney JW, Ridgway EC, Larsen PR, Lechan RM, Bianco AC. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology. 2006;147(4):1735–1743 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

