Interleukin-25-mediated resistance against intestinal trematodes does not depend on the generation of Th2 responses
- PMID: 33276813
- PMCID: PMC7716497
- DOI: 10.1186/s13071-020-04467-7
Interleukin-25-mediated resistance against intestinal trematodes does not depend on the generation of Th2 responses
Abstract
Background: The cytokine interleukin-25 (IL-25) is recognized as the most relevant initiator of protective T helper 2 (Th2) responses in intestinal helminth infections. This cytokine induces resistance against several species of intestinal helminths, including the trematode Echinostoma caproni. E. caproni has been extensively used as an experimental model to study the factors determining resistance to intestinal infections. In the study reported here, we assessed the role of IL-25 in the generation of resistance in mice infected with E. caproni.
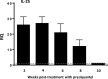
Methods: The factors that determine the production of IL-25 in mice experimentally infected with E. caproni were determined, as were the consequences of IL-25 production in terms of polarization of the immune response and resistance to infection.
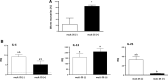
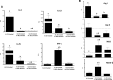
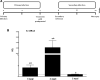
Results: Our results show that the role of IL-25 in the polarization of the immune response differs between the primary and secondary immune responses. IL-25 is required for the development of a Th2 phenotype in primary E. caproni infections, but it can also promote the differentiation to Th2 memory cell subsets that enhance type-2 immunity in memory responses. However, the development of Th2 responses does not induce resistance to infection. The Th2 phenotype does not elicit resistance, and IL-25 is responsible for the resistance regardless of its type-2 cytokine activity and activation of signal transducer and activator of transcription (STAT6). Alternative activation of macrophages induced by IL-25 can be implicated in the resistance to infection.
Conclusions: In contrast to primary infection, secondary infection elicits a type-2 immune response even in the absence of IL-25 expression. Despite the development of a type-2 response, mice are susceptible to secondary infection associated with the lack of IL-25. Resistance to infection is due to the production of IL-25, which acts autonomously from Th2 response in terms of parasite clearance.
Keywords: Echinostoma caproni; Interleuquin-25; Intestinal helminth; Resistance; Th2; Trematoda.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

