Design, synthesis and antimycobacterial evaluation of novel adamantane and adamantanol analogues effective against drug-resistant tuberculosis
- PMID: 33276981
- PMCID: PMC7775894
- DOI: 10.1016/j.bioorg.2020.104486
Design, synthesis and antimycobacterial evaluation of novel adamantane and adamantanol analogues effective against drug-resistant tuberculosis
Abstract
The treacherous nature of tuberculosis (TB) combined with the ubiquitous presence of the drug-resistant (DR) forms pose this disease as a growing public health menace. Therefore, it is imperative to develop new chemotherapeutic agents with a novel mechanism of action to circumvent the cross-resistance problems. The unique architecture of the Mycobacterium tuberculosis (M. tb) outer envelope plays a predominant role in its pathogenesis, contributing to its intrinsic resistance against available therapeutic agents. The mycobacterial membrane protein large 3 (MmpL3), which is a key player in forging the M. tb rigid cell wall, represents an emerging target for TB drug development. Several indole-2-carboxamides were previously identified in our group as potent anti-TB agents that act as inhibitor of MmpL3 transporter protein. Despite their highly potent in vitro activities, the lingering Achilles heel of these indoleamides can be ascribed to their high lipophilicity as well as low water solubility. In this study, we report our attempt to improve the aqueous solubility of these indole-2-carboxamides while maintaining an adequate lipophilicity to allow effective M. tb cell wall penetration. A more polar adamantanol moiety was incorporated into the framework of several indole-2-carboxamides, whereupon the corresponding analogues were tested for their anti-TB activity against drug-sensitive (DS) M. tb H37Rv strain. Three adamantanol derivatives 8i, 8j and 8l showed nearly 2- and 4-fold higher activity (MIC = 1.32 - 2.89 µM) than ethambutol (MIC = 4.89 µM). Remarkably, the most potent adamantanol analogue 8j demonstrated high selectivity towards DS and DR M. tb strains over mammalian cells [IC50 (Vero cells) ≥ 169 µM], evincing its lack of cytotoxicity. The top eight active compounds 8b, 8d, 8f, 8i, 8j, 8k, 8l and 10a retained their in vitro potency against DR M. tb strains and were docked into the MmpL3 active site. The most potent adamantanol/adamantane-based indoleamides 8j/8k displayed a two-fold surge in potency against extensively DR (XDR) M. tb strains with MIC values of 0.66 and 0.012 µM, respectively. The adamantanol-containing indole-2-carboxamides exhibited improved water solubility both in silico and experimentally, relative to the adamantane counterparts. Overall, the observed antimycobacterial and physicochemical profiles support the notion that adamantanol moiety is a suitable replacement to the adamantane scaffold within the series of indole-2-carboxamide-based MmpL3 inhibitors.
Keywords: Adamantane; Adamantanol; Cytotoxicity; Indoleamides; MDR-TB; MmpL3; Tuberculosis; Water solubility; XDR-TB.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
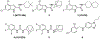


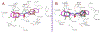


References
-
- Cambier CJ, Falkow S, Ramakrishnan L, Host evasion and exploitation schemes of Mycobacterium tuberculosis, Cell 159(7) (2014) 1497–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

