Hemodynamic and transcriptomic studies suggest early left ventricular dysfunction in a preclinical model of severe mitral regurgitation
- PMID: 33277035
- PMCID: PMC7889661
- DOI: 10.1016/j.jtcvs.2020.08.119
Hemodynamic and transcriptomic studies suggest early left ventricular dysfunction in a preclinical model of severe mitral regurgitation
Abstract
Objective: Primary mitral regurgitation is a valvular lesion in which the left ventricular ejection fraction remains preserved for long periods, delaying a clinical trigger for mitral valve intervention. In this study, we sought to investigate whether adverse left ventricular remodeling occurs before a significant fall in ejection fraction and characterize these changes.
Methods: Sixty-five rats were induced with severe mitral regurgitation by puncturing the mitral valve leaflet with a 23-G needle using ultrasound guidance. Rats underwent longitudinal cardiac echocardiography at biweekly intervals and hearts explanted at 2 weeks (n = 15), 10 weeks (n = 15), 20 weeks (n = 15), and 40 weeks (n = 15). Sixty age- and weight-matched healthy rats were used as controls. Unbiased RNA-sequencing was performed at each terminal point.
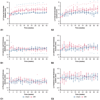
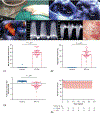
Results: Regurgitant fraction was 40.99 ± 9.40%, with pulmonary flow reversal in the experimental group, and none in the control group. Significant fall in ejection fraction occurred at 14 weeks after mitral regurgitation induction. However, before 14 weeks, end-diastolic volume increased by 93.69 ± 52.38% (P < .0001 compared with baseline), end-systolic volume increased by 118.33 ± 47.54% (P < .0001 compared with baseline), and several load-independent pump function indices were reduced. Transcriptomic data at 2 and 10 weeks before fall in ejection fraction indicated up-regulation of myocyte remodeling and oxidative stress pathways, whereas those at 20 and 40 weeks indicated extracellular matrix remodeling.
Conclusions: In this rodent model of mitral regurgitation, left ventricular ejection fraction was preserved for a long duration, yet rapid and severe left ventricular dilatation, and biological remodeling occurred before a clinically significant fall in ejection fraction.
Keywords: ejection fraction; heart murmur; mitral regurgitation; mitral valve prolapse; neochordoplasty; primary mitral regurgitation; ventricular remodeling.
Copyright © 2020 The American Association for Thoracic Surgery. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
M.P. received consulting fees from Heart Repair Technologies, Inc, which did not have any role in this study. All other authors reported no conflicts of interest.
The
Figures









Comment in
-
Commentary: Can indications for asymptomatic mitral regurgitation derive from ratatouille or should we stew on it?J Thorac Cardiovasc Surg. 2021 Mar;161(3):978-979. doi: 10.1016/j.jtcvs.2020.09.006. Epub 2020 Sep 4. J Thorac Cardiovasc Surg. 2021. PMID: 33008582 No abstract available.
-
Commentary: A step towards better understanding severe mitral regurgitation.J Thorac Cardiovasc Surg. 2021 Mar;161(3):977-978. doi: 10.1016/j.jtcvs.2020.09.033. Epub 2020 Sep 12. J Thorac Cardiovasc Surg. 2021. PMID: 33012540 No abstract available.
-
Commentary: The mitral matrix.J Thorac Cardiovasc Surg. 2021 Mar;161(3):979-980. doi: 10.1016/j.jtcvs.2020.09.087. Epub 2020 Sep 28. J Thorac Cardiovasc Surg. 2021. PMID: 33127087 No abstract available.
References
-
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. - PubMed
-
- Braunwald E, Welch GH, Sarnoff SJ. Hemodynamic effects of quantitativelyvaried experimental mitral regurgitation. Circ Res. 1957;5:539–45. - PubMed
-
- Carabello BA, Crawford FA. Valvular heart disease. N Engl J Med. 1997;337: 32–41. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

