Next-Generation CRISPR Technologies and Their Applications in Gene and Cell Therapy
- PMID: 33277043
- PMCID: PMC8166939
- DOI: 10.1016/j.tibtech.2020.10.010
Next-Generation CRISPR Technologies and Their Applications in Gene and Cell Therapy
Abstract
The emergence of clustered regularly interspaced short palindromic repeat (CRISPR) nucleases has transformed biotechnology by providing an easy, efficient, and versatile platform for editing DNA. However, traditional CRISPR-based technologies initiate editing by activating DNA double-strand break (DSB) repair pathways, which can cause adverse effects in cells and restrict certain therapeutic applications of the technology. To this end, several new CRISPR-based modalities have been developed that are capable of catalyzing editing without the requirement for a DSB. Here, we review three of these technologies: base editors, prime editors, and RNA-targeting CRISPR-associated protein (Cas)13 effectors. We discuss their strengths compared to traditional gene-modifying systems, we highlight their emerging therapeutic applications, and we examine challenges facing their safe and effective clinical implementation.
Keywords: CRISPR; CRISPR-Cas13; base editing; gene therapy; prime editing.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures


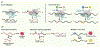
References
-
- Kim H and Kim JS (2014) A guide to genome engineering with programmable nucleases. Nat Rev Genet 15 (5), 321–34. - PubMed
-
- Wyman C and Kanaar R (2006) DNA double-strand break repair: all’s well that ends well. Annu Rev Genet 40, 363–83. - PubMed
-
- Urnov FD et al. (2010) Genome editing with engineered zinc finger nucleases. Nat Rev Genet 11 (9), 636–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

