Lipoprotein Lipase and Its Regulators: An Unfolding Story
- PMID: 33277156
- PMCID: PMC8627828
- DOI: 10.1016/j.tem.2020.11.005
Lipoprotein Lipase and Its Regulators: An Unfolding Story
Abstract
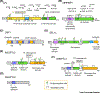
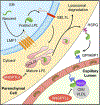
Lipoprotein lipase (LPL) is one of the most important factors in systemic lipid partitioning and metabolism. It mediates intravascular hydrolysis of triglycerides packed in lipoproteins such as chylomicrons and very-low-density lipoprotein (VLDL). Since its initial discovery in the 1940s, its biology and pathophysiological significance have been well characterized. Nonetheless, several studies in the past decade, with recent delineation of LPL crystal structure and the discovery of several new regulators such as angiopoietin-like proteins (ANGPTLs), glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1), lipase maturation factor 1 (LMF1) and Sel-1 suppressor of Lin-12-like 1 (SEL1L), have completely transformed our understanding of LPL biology.
Keywords: ANGPTL; GPIHBP1; LMF1; LPL; SEL1L; endoplasmic reticulum; endothelium; hyperlipidemia.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
References
-
- Hahn PF, Abolishment of alimentary lipemia following injection of heparin. science, 1943. 98(2531): p. 19–20. - PubMed
-
- Korn ED, Clearing factor, a heparin-activated lipoprotein lipase I. Isolation and characterization of the enzyme from normal rat heart. Journal of Biological Chemistry, 1955. 215(1): p. 1–14. - PubMed
-
- Korn ED, Clearing factor, a heparin-activated lipoprotein lipase II. Substrate specificity and activation of coconut oil. Journal of Biological Chemistry, 1955. 215(1): p. 15–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

