Reliable, low-cost, fully integrated hydration sensors for monitoring and diagnosis of inflammatory skin diseases in any environment
- PMID: 33277260
- PMCID: PMC7821898
- DOI: 10.1126/sciadv.abd7146
Reliable, low-cost, fully integrated hydration sensors for monitoring and diagnosis of inflammatory skin diseases in any environment
Abstract
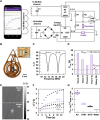
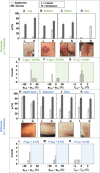
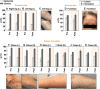
Present-day dermatological diagnostic tools are expensive, time-consuming, require substantial operational expertise, and typically probe only the superficial layers of skin (~15 μm). We introduce a soft, battery-free, noninvasive, reusable skin hydration sensor (SHS) adherable to most of the body surface. The platform measures volumetric water content (up to ~1 mm in depth) and wirelessly transmits data to any near-field communication-compatible smartphone. The SHS is readily manufacturable, comprises unique powering and encapsulation strategies, and achieves high measurement precision (±5% volumetric water content) and resolution (±0.015°C skin surface temperature). Validation on n = 16 healthy/normal human participants reveals an average skin water content of ~63% across multiple body locations. Pilot studies on patients with atopic dermatitis (AD), psoriasis, urticaria, xerosis cutis, and rosacea highlight the diagnostic capability of the SHS (P AD = 0.0034) and its ability to study impact of topical treatments on skin diseases.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



Similar articles
-
Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity.J Clin Med. 2021 Jan 19;10(2):359. doi: 10.3390/jcm10020359. J Clin Med. 2021. PMID: 33477944 Free PMC article.
-
Atopic xerosis: employment of noninvasive biophysical instrumentation for the functional analyses of the mildly abnormal stratum corneum and for the efficacy assessment of skin care products.J Cosmet Dermatol. 2006 Jun;5(2):140-9. doi: 10.1111/j.1473-2165.2006.00241.x. J Cosmet Dermatol. 2006. PMID: 17173589 Review.
-
Stretchable, Skin-Attachable Electronics with Integrated Energy Storage Devices for Biosignal Monitoring.Acc Chem Res. 2019 Jan 15;52(1):91-99. doi: 10.1021/acs.accounts.8b00508. Epub 2018 Dec 26. Acc Chem Res. 2019. PMID: 30586283 Review.
-
Recent studies of cutaneous nociception in atopic and non-atopic subjects.J Dermatol. 1999 Feb;26(2):77-86. doi: 10.1111/j.1346-8138.1999.tb03516.x. J Dermatol. 1999. PMID: 10091477 Review.
-
Functional analyses of the superficial stratum corneum in atopic xerosis.Arch Dermatol. 1991 Nov;127(11):1689-92. Arch Dermatol. 1991. PMID: 1952974
Cited by
-
Skin sensing and wearable technology as tools to measure atopic dermatitis severity.Skin Health Dis. 2024 Aug 15;4(5):e449. doi: 10.1002/ski2.449. eCollection 2024 Oct. Skin Health Dis. 2024. PMID: 39355726 Free PMC article. Review.
-
Transforming Healthcare: Intelligent Wearable Sensors Empowered by Smart Materials and Artificial Intelligence.Adv Mater. 2025 May;37(21):e2500412. doi: 10.1002/adma.202500412. Epub 2025 Apr 1. Adv Mater. 2025. PMID: 40167502 Free PMC article. Review.
-
Hydrogel-Based Biosensors for Effective Therapeutics.Gels. 2023 Jul 4;9(7):545. doi: 10.3390/gels9070545. Gels. 2023. PMID: 37504424 Free PMC article. Review.
-
Near-Field Communication in Biomedical Applications.Sensors (Basel). 2021 Jan 20;21(3):703. doi: 10.3390/s21030703. Sensors (Basel). 2021. PMID: 33498589 Free PMC article. Review.
-
An epidermal serine sensing system for skin healthcare.Nat Commun. 2025 Mar 18;16(1):2681. doi: 10.1038/s41467-025-58147-0. Nat Commun. 2025. PMID: 40102486 Free PMC article.
References
-
- Lim H. W., Collins S. A. B., Resneck J. S. Jr., Bolognia J. L., Hodge J. A., Rohrer T. A., Van Beek M. J., Margolis D. J., Sober A. J., Weinstock M. A., Nerenz D. R., Smith Begolka W., Moyano J. V., The burden of skin disease in the United States. J. Am. Acad. Dermatol. 76, 958–972.e2 (2017). - PubMed
-
- Lowell B. A., Froelich C. W., Federman D. G., Kirsner R. S., Dermatology in primary care: Prevalence and patient disposition. J. Am. Acad. Dermatol. 45, 250–255 (2001). - PubMed
-
- Basarab T., Munn S. E., Jones R. R., Diagnostic accuracy and appropriateness of general practitioner referrals to a dermatology out-patient clinic. Br. J. Dermatol. 135, 70–73 (1996). - PubMed
-
- Wagner R. F. Jr., Wagner D., Tomich J. M., Wagner K. D., Grande D. J., Diagnoses of skin disease: Dermatologists vs. nondermatologists. J. Dermatol. Surg. Oncol. 11, 476–479 (1985). - PubMed
Publication types
LinkOut - more resources
Full Text Sources

