MDMX Recruits UbcH5c to Facilitate MDM2 E3 Ligase Activity and Subsequent p53 Degradation In Vivo
- PMID: 33277368
- PMCID: PMC8026549
- DOI: 10.1158/0008-5472.CAN-20-0790
MDMX Recruits UbcH5c to Facilitate MDM2 E3 Ligase Activity and Subsequent p53 Degradation In Vivo
Abstract
MDM2 regulates p53 degradation by functioning as an E3 ubiquitin ligase. The role of MDMX, an MDM2 homolog that lacks E3 ligase activity, in the regulation of p53 degradation remains incompletely understood and sometime controversial. This confusion is due at least in part to studies of p53 degradation mainly carried out in in vitro settings, as elimination of either MDM2 or MDMX from mice results in p53-dependent embryonic lethality, thus obfuscating in vivo studies of the individual roles of MDM2 and MDMX in p53 degradation. To overcome this problem, we generated mice expressing an inducible p53 allele under various MDM2 and MDMX deletion and mutation statuses and studied in vivo p53 degradation. Degradation of p53 in vivo was largely prevented in mice and mouse embryonic fibroblast retaining MDM2 but lacking MDMX. Although MDM2 and MDMX interacted with p53 in the absence of each other, they bound p53 more efficiently as a heterodimer. MDMX, but not MDM2, interacted with ubiquitin-conjugating enzyme UbcH5c, an interaction that was essential for MDMX to enable MDM2 E3 ligase activity for p53 degradation. Grafting the C-terminal residues of MDMX to the C-terminus of MDM2 allowed MDM2 to interact with UbcH5c and enhanced MDM2-mediated p53 degradation in the absence of MDMX. Together, these data indicate that MDMX plays an essential role for p53 degradation in vivo by recruiting UbcH5c to facilitate MDM2 E3 ligase function. SIGNIFICANCE: This study provides the first in vivo evidence of MDMX facilitating MDM2-mediated p53 degradation, clarifying its role in the regulation of this critical tumor suppressor.
©2020 American Association for Cancer Research.
Conflict of interest statement
CONFLICTS OF INTEREST STATEMENT
The authors declare no potential conflicts of interest.
Figures





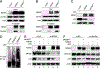
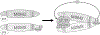
Comment in
-
Mdm2 and MdmX: Partners in p53 Destruction.Cancer Res. 2021 Apr 1;81(7):1633-1634. doi: 10.1158/0008-5472.CAN-21-0145. Cancer Res. 2021. PMID: 34003788
Similar articles
-
MDMX is essential for the regulation of p53 protein levels in the absence of a functional MDM2 C-terminal tail.BMC Mol Cell Biol. 2021 Sep 22;22(1):46. doi: 10.1186/s12860-021-00385-3. BMC Mol Cell Biol. 2021. PMID: 34551723 Free PMC article.
-
p53 regulation: teamwork between RING domains of Mdm2 and MdmX.Cell Cycle. 2011 Dec 15;10(24):4225-9. doi: 10.4161/cc.10.24.18662. Epub 2011 Dec 15. Cell Cycle. 2011. PMID: 22134240 Review.
-
MdmX protein is essential for Mdm2 protein-mediated p53 polyubiquitination.J Biol Chem. 2011 Jul 8;286(27):23725-34. doi: 10.1074/jbc.M110.213868. Epub 2011 May 13. J Biol Chem. 2011. PMID: 21572037 Free PMC article.
-
MDM2, MDMX, and p73 regulate cell-cycle progression in the absence of wild-type p53.Proc Natl Acad Sci U S A. 2021 Nov 2;118(44):e2102420118. doi: 10.1073/pnas.2102420118. Proc Natl Acad Sci U S A. 2021. PMID: 34716260 Free PMC article.
-
Regulation of p53: a collaboration between Mdm2 and Mdmx.Oncotarget. 2012 Mar;3(3):228-35. doi: 10.18632/oncotarget.443. Oncotarget. 2012. PMID: 22410433 Free PMC article. Review.
Cited by
-
Expanding Roles of the E2F-RB-p53 Pathway in Tumor Suppression.Biology (Basel). 2023 Dec 11;12(12):1511. doi: 10.3390/biology12121511. Biology (Basel). 2023. PMID: 38132337 Free PMC article. Review.
-
Mitochondrial STAT3 exacerbates LPS-induced sepsis by driving CPT1a-mediated fatty acid oxidation.Theranostics. 2022 Jan 1;12(2):976-998. doi: 10.7150/thno.63751. eCollection 2022. Theranostics. 2022. PMID: 34976224 Free PMC article.
-
Exploring the role of ferroptosis-related genes as biomarkers in acute kidney injury.PLoS One. 2024 Jul 23;19(7):e0307472. doi: 10.1371/journal.pone.0307472. eCollection 2024. PLoS One. 2024. PMID: 39042632 Free PMC article.
-
Clinical and Immunological Effects of p53-Targeting Vaccines.Front Cell Dev Biol. 2021 Nov 3;9:762796. doi: 10.3389/fcell.2021.762796. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34805170 Free PMC article. Review.
-
Qishen Granule Protects against Doxorubicin-Induced Cardiotoxicity by Coordinating MDM2-p53-Mediated Mitophagy and Mitochondrial Biogenesis.Oxid Med Cell Longev. 2022 Sep 6;2022:4344677. doi: 10.1155/2022/4344677. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36120600 Free PMC article.
References
-
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell 2009;137(3):413–31. - PubMed
-
- Geyer RK, Yu ZK, Maki CG. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nature cell biology 2000;2:569–73. - PubMed
-
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992;69(7):1237–45. - PubMed
-
- Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 1995;378:206–08. - PubMed
-
- Luna RM, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995;378:203–06. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

