Coiled-coil domain containing 50-V2 protein positively regulates neurite outgrowth
- PMID: 33277610
- PMCID: PMC7718278
- DOI: 10.1038/s41598-020-78304-3
Coiled-coil domain containing 50-V2 protein positively regulates neurite outgrowth
Abstract
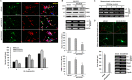
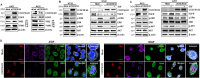
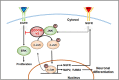
The coiled-coil domain containing 50 (CCDC50) protein is a phosphotyrosine-dependent signalling protein stimulated by epidermal growth factor. It is highly expressed in neuronal cells in the central nervous system; however, the roles of CCDC50 in neuronal development are largely unknown. In this study, we showed that the depletion of CCDC50-V2 impeded the neuronal development process, including arbor formation, spine density development, and axonal outgrowth, in primary neurons. Mechanistic studies revealed that CCDC50-V2 positively regulated the nerve growth factor receptor, while it downregulated the epidermal growth factor receptor pathway. Importantly, JNK/c-Jun activation was found to be induced by the CCDC50-V2 overexpression, in which the interaction between CCDC50-V2 and JNK2 was also observed. Overall, the present study demonstrates a novel mechanism of CCDC50 function in neuronal development and provides new insight into the link between CCDC50 function and the aetiology of neurological disorders.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

