Amotosalen and ultraviolet A light treatment efficiently inactivates severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human plasma
- PMID: 33277935
- PMCID: PMC8359189
- DOI: 10.1111/vox.13043
Amotosalen and ultraviolet A light treatment efficiently inactivates severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human plasma
Abstract
Background and objectives: During the ongoing pandemic of COVID-19, SARS-CoV-2 RNA was detected in plasma and platelet products from asymptomatic blood donors, raising concerns about potential risk of transfusion transmission, also in the context of the current therapeutic approach utilizing plasma from convalescent donors. The objective of this study was to assess the efficacy of amotosalen/UVA light treatment to inactivate SARS-CoV-2 in human plasma to reduce the risk of potential transmission through blood transfusion.
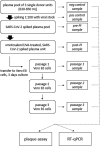
Methods: Pools of three whole-blood-derived human plasma units (630-650 ml) were inoculated with a clinical SARS-CoV-2 isolate. Spiked units were treated with amotosalen/UVA light (INTERCEPT Blood System™) to inactivate SARS-CoV-2. Infectious titres and genomic viral load were assessed by plaque assay and real-time quantitative PCR. Inactivated samples were subject to three successive passages on permissive tissue culture to exclude the presence of replication-competent viral particles.
Results: Inactivation of infectious viral particles in spiked plasma units below the limit of detection was achieved by amotosalen/UVA light treatment with a mean log reduction of >3·32 ± 0·2. Passaging of inactivated samples on permissive tissue showed no viral replication even after 9 days of incubation and three passages, confirming complete inactivation. The treatment also inhibited NAT detection by nucleic acid modification with a mean log reduction of 2·92 ± 0·87 PFU genomic equivalents.
Conclusion: Amotosalen/UVA light treatment of SARS-CoV-2 spiked human plasma units efficiently and completely inactivated >3·32 ± 0·2 log of SARS-CoV-2 infectivity, showing that such treatment could minimize the risk of transfusion-related SARS-CoV-2 transmission.
Keywords: SARS-CoV-2; amotosalen/UVA; pathogen inactivation; plasma.
© 2020 The Authors. Vox Sanguinis published by John Wiley & Sons Ltd on behalf of International Society of Blood Transfusion.
Conflict of interest statement
There is no conflict of interest identified.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

