Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub
- PMID: 33278301
- PMCID: PMC7724912
- DOI: 10.1182/bloodadvances.2020003170
Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub
Abstract
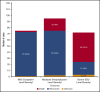
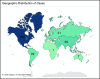
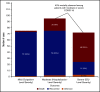
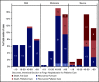
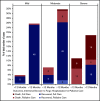
Coronavirus disease 2019 (COVID-19) is an illness resulting from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that emerged in late 2019. Patients with cancer, and especially those with hematologic malignancies, may be at especially high risk of adverse outcomes, including mortality resulting from COVID-19 infection. The ASH Research Collaborative COVID-19 Registry for Hematology was developed to study features and outcomes of COVID-19 infection in patients with underlying blood disorders, such as hematologic malignancies. At the time of this report, data from 250 patients with blood cancers from 74 sites around the world had been entered into the registry. The most commonly represented malignancies were acute leukemia (33%), non-Hodgkin lymphoma (27%), and myeloma or amyloidosis (16%). Patients presented with a myriad of symptoms, most frequently fever (73%), cough (67%), dyspnea (50%), and fatigue (40%). Use of COVID-19-directed therapies, such as hydroxychloroquine (n = 76) or azithromycin (n = 59), was common. Overall mortality was 28%. Patients with a physician-estimated prognosis from the underlying hematologic malignancy of <12 months at the time of COVID-19 diagnosis and those with relapsed/refractory disease experienced a higher proportion of moderate/severe COVID-19 disease and death. In some instances, death occurred after a decision was made to forgo intensive care unit admission in favor of a palliative approach. Taken together, these data support the emerging consensus that patients with hematologic malignancies experience significant morbidity and mortality resulting from COVID-19 infection. Batch submissions from sites with high incidence of COVID-19 infection are planned to support future analyses.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict--interst disclosure: D.S.N. received research support from Pharmacyclics; and holds stock in Madrigal. W.A.W. received research support from Pfizer; provided consulting for Teladoc; was an advisor for Koneksa Health and Elektra Labs; and received honoraria from the ASH Research Collaborative. L.K.H. received research support from Gilead. N.A.P. provided consulting for AstraZeneca, BMS, Merck, Genentech, Amgen, Eli Lilly, and G1 therapeutics. M.S.T. received research support from AbbVie, Cellerant, Orsenix, ADC Therapeutics, Biosight, Glycomimetics, Rafael Pharmaceuticals, and Amgen; served on advisory boards for AbbVie, BioLineRx, Daiichi-Sankyo, Orsenix, KAHR, Rigel, Nohla, Delta Fly Pharma, Tetraphase, Oncolyze, Jazz Pharma, Roche, Biosight, and Novartis; and received royalties from UpToDate. C.M.N. provided consulting for Celgene and Novartis. A.D.G. served on advisory boards for AbbVie, Aptose, Celgene, Daiichi Sankyo, and Genentech; received research support from AbbVie, ADC Therapeutics, Aprea, Aptose, AROG, Celularity, Daiici Sankyo, and Pfizer; and received honoraria from Dava Oncology. L.H.S. received consulting or honoraria from Roche/Genentech, AbbVie, Amgen, Apobiologix, AstraZeneca, Acerta, Celgene, Gilead, Janssen, Kite Karyopharm, Lundbeck, Merck, Morphosys, Seattle Genetics, Teva, Takeda, TG Therapeutics, and Verastem. The remaining authors declare no competing financial interests.
Figures
References
-
- World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19 – 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re.... Accessed 23 September 2020.
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

