A Randomized Phase I Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Recombinant Erwinia Asparaginase (JZP-458) in Healthy Adult Volunteers
- PMID: 33278328
- PMCID: PMC8212713
- DOI: 10.1111/cts.12947
A Randomized Phase I Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Recombinant Erwinia Asparaginase (JZP-458) in Healthy Adult Volunteers
Abstract
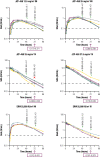
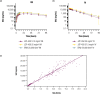
L-asparaginase has been an important component of acute lymphoblastic leukemia (ALL) therapy for over 40 years, and is standard therapy during ALL induction and consolidation treatment. L-asparaginases are immunogenic and can induce hypersensitivity reactions; inability to receive asparaginase has been associated with poor patient outcomes. There are L-asparaginases of varied bacterial origins, with the most commonly used being Escherichia coli (E. coli); therefore, to ensure that patients who develop hypersensitivity to E. coli-derived asparaginases receive an adequate therapeutic course, alternative preparations are warranted. JZP-458 is a recombinant Erwinia asparaginase produced using a novel Pseudomonas fluorescens expression platform that yields an enzyme with no immunologic cross-reactivity to E. coli-derived asparaginases. To evaluate the safety, tolerability, and pharmacokinetics (PK) of a single dose of JZP-458, a randomized, single-center, open-label, phase I study was conducted with JZP-458 given via i.m. injection or i.v. infusion to healthy adult volunteers. At the highest doses tested for each route of administration (i.e., 25 mg/m2 i.m. and 37.5 mg/m2 i.v.), JZP-458 achieved serum asparaginase activity (SAA) levels ≥ 0.1 IU/mL at 72 hours postdose for 100% of volunteers. Bioavailability for i.m. JZP-458 was estimated at 36.8% based on SAA data. All dose levels were well-tolerated, with no unanticipated adverse events (AEs), no serious AEs, and no grade 3 or higher AEs. Based on PK and safety data, the recommended JZP-458 starting dose for the pivotal phase II/III study in adult and pediatric patients is 25 mg/m2 i.m. and 37.5 mg/m2 i.v. on a Monday/Wednesday/Friday dosing schedule.
© 2020 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
T.L. is an employee of and holds stock ownership and/or stock options in Jazz Pharmaceuticals. M.H.‐I. is an employee of QPS Miami Research Associates. A.R. is an employee of QPS Miami Research Associates. J.J. is a contract employee of Jazz Pharmaceuticals. R.C. is an employee of and holds stock ownership and/or stock options in Jazz Pharmaceuticals. J.A.S. is an employee of and holds stock ownership and/or stock options in Jazz Pharmaceuticals. M.R.C. is an employee of and holds stock ownership and/or stock options in Jazz Pharmaceuticals.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

