Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes
- PMID: 33278357
- PMCID: PMC7674074
- DOI: 10.1016/j.cell.2020.11.025
Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes
Abstract
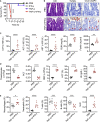
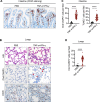
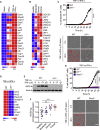
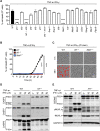
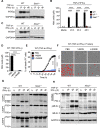
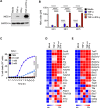
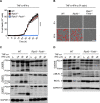
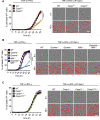
COVID-19 is characterized by excessive production of pro-inflammatory cytokines and acute lung damage associated with patient mortality. While multiple inflammatory cytokines are produced by innate immune cells during SARS-CoV-2 infection, we found that only the combination of TNF-α and IFN-γ induced inflammatory cell death characterized by inflammatory cell death, PANoptosis. Mechanistically, TNF-α and IFN-γ co-treatment activated the JAK/STAT1/IRF1 axis, inducing nitric oxide production and driving caspase-8/FADD-mediated PANoptosis. TNF-α and IFN-γ caused a lethal cytokine shock in mice that mirrors the tissue damage and inflammation of COVID-19, and inhibiting PANoptosis protected mice from this pathology and death. Furthermore, treating with neutralizing antibodies against TNF-α and IFN-γ protected mice from mortality during SARS-CoV-2 infection, sepsis, hemophagocytic lymphohistiocytosis, and cytokine shock. Collectively, our findings suggest that blocking the cytokine-mediated inflammatory cell death signaling pathway identified here may benefit patients with COVID-19 or other infectious and autoinflammatory diseases by limiting tissue damage/inflammation.
Keywords: COVID-19; IFN-γ; PANoptosis; SARS-CoV-2; TNF-α; apoptosis; cytokine storm; inflammation; necroptosis; pyroptosis.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests St. Jude Children’s Research hospital filed a provisional patent application on TNF-α and IFN-γ signaling described in this study, listing R.K. and T.-D.K. as inventors (serial no. 63/106,012).
Figures





Update of
-
Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes.bioRxiv [Preprint]. 2020 Nov 13:2020.10.29.361048. doi: 10.1101/2020.10.29.361048. bioRxiv. 2020. Update in: Cell. 2021 Jan 7;184(1):149-168.e17. doi: 10.1016/j.cell.2020.11.025. PMID: 33140051 Free PMC article. Updated. Preprint.
References
-
- Albina J.E., Reichner J.S. Role of nitric oxide in mediation of macrophage cytotoxicity and apoptosis. Cancer Metastasis Rev. 1998;17:39–53. - PubMed
-
- Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N., Justet A. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann. Rheum. Dis. 2020;79:1381–1382. - PubMed
-
- Bailey A., Pope T.W., Moore S.A., Campbell C.L. The tragedy of TRIUMPH for nitric oxide synthesis inhibition in cardiogenic shock: where do we go from here? Am. J. Cardiovasc. Drugs. 2007;7:337–345. - PubMed
-
- Belkhelfa M., Rafa H., Medjeber O., Arroul-Lammali A., Behairi N., Abada-Bendib M., Makrelouf M., Belarbi S., Masmoudi A.N., Tazir M., Touil-Boukoffa C. IFN-γ and TNF-α are involved during Alzheimer disease progression and correlate with nitric oxide production: a study in Algerian patients. J. Interferon Cytokine Res. 2014;34:839–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

