Rescue from Pseudomonas aeruginosa Airway Infection via Stem Cell Transplantation
- PMID: 33279724
- PMCID: PMC7935663
- DOI: 10.1016/j.ymthe.2020.12.003
Rescue from Pseudomonas aeruginosa Airway Infection via Stem Cell Transplantation
Abstract
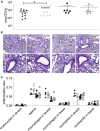
Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which lead to impaired ion transport in epithelial cells. Although lung failure due to chronic infection is the major comorbidity in individuals with cystic fibrosis, the role of CFTR in non-epithelial cells has not been definitively resolved. Given the important role of host defense cells, we evaluated the Cftr deficiency in pulmonary immune cells by hematopoietic stem cell transplantation in cystic fibrosis mice. We transplanted healthy bone marrow stem cells and could reveal a stable chimerism of wild-type cells in peripheral blood. The outcome of stem cell transplantation and the impact of healthy immune cells were evaluated in acute Pseudomonas aeruginosa airway infection. In this study, mice transplanted with wild-type cells displayed better survival, lower lung bacterial numbers, and a milder disease course. This improved physiology of infected mice correlated with successful intrapulmonary engraftment of graft-derived alveolar macrophages, as seen by immunofluorescence microscopy and flow cytometry of graft-specific leucocyte surface marker CD45 and macrophage marker CD68. Given the beneficial effect of hematopoietic stem cell transplantation and stable engraftment of monocyte-derived CD68-positive macrophages, we conclude that replacement of mutant Cftr macrophages attenuates airway infection in cystic fibrosis mice.
Keywords: Pseudomonas aeruginosa; airway macrophages; chronic lung infection; cystic fibrosis; hematopoietic stem cell transplantation; mouse model; non-epithelial cells.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Elborn J.S. Cystic fibrosis. Lancet. 2016;388:2519–2531. - PubMed
-
- Mall M.A., Hartl D. CFTR: cystic fibrosis and beyond. Eur. Respir. J. 2014;44:1042–1054. - PubMed
-
- Guan S., Munder A., Hedtfeld S., Braubach P., Glage S., Zhang L., Lienenklaus S., Schultze A., Hasenpusch G., Garrels W. Self-assembled peptide-poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat. Nanotechnol. 2019;14:287–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

