Prophylactic intranasal administration of a TLR2/6 agonist reduces upper respiratory tract viral shedding in a SARS-CoV-2 challenge ferret model
- PMID: 33279857
- PMCID: PMC7711201
- DOI: 10.1016/j.ebiom.2020.103153
Prophylactic intranasal administration of a TLR2/6 agonist reduces upper respiratory tract viral shedding in a SARS-CoV-2 challenge ferret model
Abstract
Background: The novel human coronavirus SARS-CoV-2 is a major ongoing global threat with huge economic burden. Like all respiratory viruses, SARS-CoV-2 initiates infection in the upper respiratory tract (URT). Infected individuals are often asymptomatic, yet highly infectious and readily transmit virus. A therapy that restricts initial replication in the URT has the potential to prevent progression of severe lower respiratory tract disease as well as limiting person-to-person transmission.
Methods: SARS-CoV-2 Victoria/01/2020 was passaged in Vero/hSLAM cells and virus titre determined by plaque assay. Challenge virus was delivered by intranasal instillation to female ferrets at 5.0 × 106 pfu/ml. Treatment groups received intranasal INNA-051, developed by Ena Respiratory. SARS-CoV-2 RNA was detected using the 2019-nCoV CDC RUO Kit and QuantStudio™ 7 Flex Real-Time PCR System. Histopathological analysis was performed using cut tissues stained with haematoxylin and eosin (H&E).
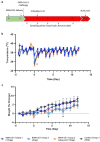
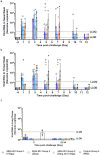
Findings: We show that prophylactic intra-nasal administration of the TLR2/6 agonist INNA-051 in a SARS-CoV-2 ferret infection model effectively reduces levels of viral RNA in the nose and throat. After 5 days post-exposure to SARS-CoV-2, INNA-051 significantly reduced virus in throat swabs (p=<0.0001) by up to a 24 fold (96% reduction) and in nasal wash (p=0.0107) up to a 15 fold (93% reduction) in comparison to untreated animals.
Interpretation: The results of our study support clinical development of a therapy based on prophylactic TLR2/6 innate immune activation in the URT, to reduce SARS-CoV-2 transmission and provide protection against COVID-19.
Funding: This work was funded by Ena Respiratory, Melbourne, Australia.
Keywords: COVID-19; Ferret; INNA-051; SARS-CoV-2; TLR-2; Viral shedding.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interests Authors report grants from Ena Respiratory, during the conduct of the study. W. Zeng and D.C. Jackson reports grants from Ena Therapeutics, during the conduct of the study. D. Tsitoura, C. Demaison, F. Mercuri, I. Holmes and N.W. Bartlett reports personal fees and other from Ena Therapeutics, outside the submitted work. D.C. Jackson, W. Zeng and B.Y. Chua reports other from Ena Therapeutics, outside the submitted work. Dr. Holmes reports personal fees from Ena Therapeutics, outside the submitted work. In addition, D. Tsitoura, C. Demaison and F. Mercuri have a patent AU 2020901709 pending to Ena Therapeutics. D.C Jackson, W. Zeng and C. Demaison have a patent PCT/AU2011/001225 issued to Ena Therapeutics. D.C Jackson, W. Zeng, I. Holmes and C. Demaison have a patent PCT/AU2020/050660 pending to Ena Therapeutics.
Figures
References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

