A Novel Three-Dimensional Skin Disease Model to Assess Macrophage Function in Diabetes
- PMID: 33280487
- PMCID: PMC8349718
- DOI: 10.1089/ten.TEC.2020.0263
A Novel Three-Dimensional Skin Disease Model to Assess Macrophage Function in Diabetes
Abstract
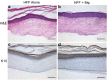
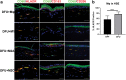
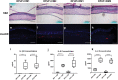
A major challenge in the management of patients suffering from diabetes is the risk of developing nonhealing foot ulcers. Most in vitro methods to screen drugs for wound healing therapies rely on conventional 2D cell cultures that do not closely mimic the complexity of the diabetic wound environment. In addition, while three-dimensional (3D) skin tissue models of human skin exist, they have not previously been adapted to incorporate patient-derived macrophages to model inflammation from these wounds. In this study, we present a 3D human skin equivalent (HSE) model incorporating blood-derived monocytes and primary fibroblasts isolated from patients with diabetic foot ulcers (DFUs). We demonstrate that the monocytes differentiate into macrophages when incorporated into HSEs and secrete a cytokine profile indicative of the proinflammatory M1 phenotype seen in DFUs. We also show how the interaction between fibroblasts and macrophages in the HSE can guide macrophage polarization. Our findings take us a step closer to creating a human, 3D skin-like tissue model that can be applied to evaluate the response of candidate compounds needed for potential new foot ulcer therapies in a more complex tissue environment that contributes to diabetic wounds. Impact statement This study is the first to incorporate disease-specific, diabetic macrophages into a three-dimensional (3D) model of human skin. We show how to fabricate skin that incorporates macrophages with disease-specific fibroblasts to guide macrophage polarization. We also show that monocytes from diabetic patients can differentiate into macrophages directly in this skin disease model, and that they secrete a cytokine profile mimicking the proinflammatory M1 phenotype seen in diabetic foot ulcers. The data presented here indicate that this 3D skin disease model can be used to study macrophage-related inflammation in diabetes and as a drug testing tool to evaluate new treatments for the disease.
Keywords: diabetes; diabetic foot ulcer; human skin equivalent; macrophage; skin model.
Conflict of interest statement
The authors state no competing financial interests exist.
Figures



References
-
- Ramsey, S.D., Newton, K., Blough, D., et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 22, 382, 1999 - PubMed
-
- Boulton, A.J., Vileikyte, L., Ragnarson-Tennvall, G., and Apelqvist, J.. The global burden of diabetic foot disease. Lancet 366, 1719, 2005 - PubMed
-
- Pham, H.T., Economides, P.A., and Veves, A.. The role of endothelial function on the foot. Microcirculation and wound healing in patients with diabetes. Clin Podiatr Med Surg 15, 85, 1998 - PubMed
-
- Breslin, S., and O'Driscoll, L.. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 18, 240, 2013 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

