A Rapidly Deployable Test Suite for Respiratory Protective Devices in the COVID-19 Pandemic
- PMID: 33281505
- PMCID: PMC7684451
- DOI: 10.1177/1535676020947284
A Rapidly Deployable Test Suite for Respiratory Protective Devices in the COVID-19 Pandemic
Abstract
Introduction: The current COVID-19 pandemic has caused large shortages in personal protective equipment, leading to hospitals buying their supplies from alternative suppliers or even reusing single-use items. Equipment from these alternative sources first needs to be tested to ensure that they properly protect the clinicians that depend on them. This work demonstrates a test suite for protective face masks that can be realized rapidly and cost effectively, using mainly off-the-shelf as well as 3D printing components.
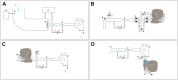
Materials and methods: The proposed test suite was designed and evaluated in order to assess its safety and proper functioning according to the criteria that are stated in the European standard norm EN149:2001+A1 7. These include a breathing resistance test, a CO2 build-up test, and a penetration test. Measurements were performed for a variety of commercially available protective face masks for validation.
Results: The results obtained with the rapidly deployable test suite agree with conventional test methods, demonstrating that this setup can be used to assess the filtering properties of protective masks when conventional equipment is not available.
Discussion: The presented test suite can serve as a starting point for the rapid deployment of more testing facilities for respiratory protective equipment. This could greatly increase the testing capacity and ultimately improve the safety of healthcare workers battling the COVID-19 pandemic.
Keywords: COVID-19; breathing resistance; carbon dioxide concentration; particle penetration; protective face masks.
© ABSA International 2020.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Use of Personal Protective Equipment Among Healthcare Workers During the First and the Second Wave of the COVID-19 Pandemic.Ann Work Expo Health. 2023 Jan 12;67(1):59-75. doi: 10.1093/annweh/wxac054. Ann Work Expo Health. 2023. PMID: 36039576 Free PMC article.
-
Assessing the filtration efficiency and regulatory status of N95s and nontraditional filtering face-piece respirators available during the COVID-19 pandemic.BMC Infect Dis. 2021 Jul 29;21(1):712. doi: 10.1186/s12879-021-06008-8. BMC Infect Dis. 2021. PMID: 34325673 Free PMC article.
-
Quantitative Fit Tested N95 Respirator-Alternatives Generated With CT Imaging and 3D Printing: A Response to Potential Shortages During the COVID-19 Pandemic.Acad Radiol. 2021 Feb;28(2):158-165. doi: 10.1016/j.acra.2020.11.005. Epub 2020 Nov 21. Acad Radiol. 2021. PMID: 33257256 Free PMC article.
-
COVID-19 coronavirus: recommended personal protective equipment for the orthopaedic and trauma surgeon.Knee Surg Sports Traumatol Arthrosc. 2020 Jun;28(6):1690-1698. doi: 10.1007/s00167-020-06022-4. Epub 2020 Apr 27. Knee Surg Sports Traumatol Arthrosc. 2020. PMID: 32342138 Free PMC article. Review.
-
Sensing and 3D printing technologies in personalized healthcare for the management of health crises including the COVID-19 outbreak.Sens Int. 2022;3:100180. doi: 10.1016/j.sintl.2022.100180. Epub 2022 May 14. Sens Int. 2022. PMID: 35601184 Free PMC article. Review.
Cited by
-
A Review of Gas Measurement Practices and Sensors for Tunnels.Sensors (Basel). 2023 Jan 17;23(3):1090. doi: 10.3390/s23031090. Sensors (Basel). 2023. PMID: 36772130 Free PMC article. Review.
-
Quality versus emergency: How good were ventilation fittings produced by additive manufacturing to address shortages during the COVID19 pandemic?PLoS One. 2022 Apr 21;17(4):e0263808. doi: 10.1371/journal.pone.0263808. eCollection 2022. PLoS One. 2022. PMID: 35446853 Free PMC article.
-
Effects of COVID-19 protective face masks and wearing durations on respiratory haemodynamic physiology and exhaled breath constituents.Eur Respir J. 2022 Sep 22;60(3):2200009. doi: 10.1183/13993003.00009-2022. Print 2022 Sep. Eur Respir J. 2022. PMID: 35169028 Free PMC article.
-
Comparison of filtration efficiency and respiratory resistance of COVID-19 protective masks by multi-national standards.Am J Infect Control. 2022 May;50(5):516-524. doi: 10.1016/j.ajic.2022.02.009. Epub 2022 Feb 11. Am J Infect Control. 2022. PMID: 35158009 Free PMC article.
-
Possible toxicity of chronic carbon dioxide exposure associated with face mask use, particularly in pregnant women, children and adolescents - A scoping review.Heliyon. 2023 Apr;9(4):e14117. doi: 10.1016/j.heliyon.2023.e14117. Epub 2023 Mar 3. Heliyon. 2023. PMID: 37057051 Free PMC article.
References
-
- Mahase E. Novel coronavirus: Australian GPs raise concerns about shortage of face masks. BMJ. 2020;368:m477. - PubMed
-
- World Health Organization. Rational Use of Personal Protective Equipment (PPE) for Coronavirus Disease (COVID-19): Interim Guidance, 19 March 2020 2020. 2020.
-
- Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic. JAMA. 2020;2019:E1–E3. - PubMed
-
- European Safety Federation. COVID19 - suspicious safety certificates for PPE. July 9, 2020. Accessed July 27, 2020. https://www.eu-esf.org/covid-19/4513-covid-19-suspicious-certificates-fo....
LinkOut - more resources
Full Text Sources
