Pleiotropic Role of Tenascin-C in Central Nervous System Diseases: From Basic to Clinical Applications
- PMID: 33281711
- PMCID: PMC7691598
- DOI: 10.3389/fneur.2020.576230
Pleiotropic Role of Tenascin-C in Central Nervous System Diseases: From Basic to Clinical Applications
Abstract
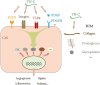
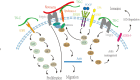
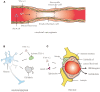
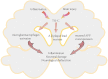
The extracellular matrix is composed of a variety of macromolecular substances secreted by cells, which form a complex network that supports and connects tissue structures, regulates the morphogenesis of tissues, and maintains the physiological activities of cells. Tenascin-C, a secreted extracellular matrix glycoprotein, is abundantly expressed after exposure to pathological stimuli. It plays an important regulatory role in brain tumors, vascular diseases, and neurodegenerative diseases by mediating inflammatory responses, inducing brain damage, and promoting cell proliferation, migration, and angiogenesis through multiple signaling pathways. Therefore, tenascin-C may become a potential therapeutic target for intracranial diseases. Here, we review and discuss the latest literature regarding tenascin-C, and we comprehensively explain the role and clinical significance of tenascin-C in intracranial diseases.
Keywords: brain injury; inflammatory; intracranial tumor; neurodegenerative diseases; tenascin-C; therapeutic target; vascular diseases.
Copyright © 2020 Hanmin, Xiangyue, Lenahan, Ling, Yibo and Yue.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

