Phytohormone-Mediated Molecular Mechanisms Involving Multiple Genes and QTL Govern Grain Number in Rice
- PMID: 33281879
- PMCID: PMC7689023
- DOI: 10.3389/fgene.2020.586462
Phytohormone-Mediated Molecular Mechanisms Involving Multiple Genes and QTL Govern Grain Number in Rice
Abstract
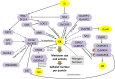
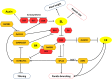
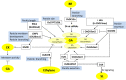
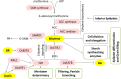
Increasing the grain number is the most direct route toward enhancing the grain yield in cereals. In rice, grain number can be amplified through increasing the shoot branching (tillering), panicle branching, panicle length, and seed set percentage. Phytohormones have been conclusively shown to control the above characteristics by regulating molecular factors and their cross-interactions. The dynamic equilibrium of cytokinin levels in both shoot and inflorescence meristems, maintained by the regulation of its biosynthesis, activation, and degradation, determines the tillering and panicle branching, respectively. Auxins and gibberellins are known broadly to repress the axillary meristems, while jasmonic acid is implicated in the determination of reproductive meristem formation. The balance of auxin, gibberellin, and cytokinin determines meristematic activities in the inflorescence. Strigolactones have been shown to repress the shoot branching but seem to regulate panicle branching positively. Ethylene, brassinosteroids, and gibberellins regulate spikelet abortion and grain filling. Further studies on the optimization of endogenous hormonal levels can help in the expansion of the grain yield potential of rice. This review focuses on the molecular machinery, involving several genes and quantitative trait loci (QTL), operational in the plant that governs hormonal control and, in turn, gets governed by the hormones to regulate grain number and yield in rice.
Keywords: grain number; panicle branching; phytohormones; rice; tiller; yield.
Copyright © 2020 Deveshwar, Prusty, Sharma and Tyagi.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

