Porphyromonas gingivalis and Lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2
- PMID: 33282294
- PMCID: PMC7685903
- DOI: 10.1002/cti2.1213
Porphyromonas gingivalis and Lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2
Abstract
Objectives: CD4+ T cells are the key to many immune-inflammatory diseases mediated by microbial disorders, especially inflammatory bowel disease (IBD). The purpose of this study was to explore how pathogenic and probiotic bacteria directly affect the T helper (Th)17 and T regulatory (Treg) cell balance among CD4+ T cells to regulate inflammation.
Methods: Porphyromonas gingivalis (Pg; ATCC 33277) and Lactobacillus rhamnosus GG (LGG; CICC 6141) were selected as representative pathogenic and probiotic bacteria, respectively. Bacterial extracts were obtained via ultrasonication and ultracentrifugation. Flow cytometry, RT-qPCR, ELISAs, immunofluorescence and a Quantibody cytokine array were used. The dextran sodium sulphate (DSS)-induced colitis model was selected for verification.
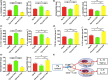
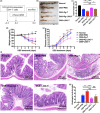
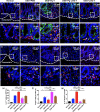
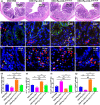
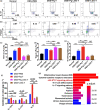
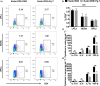
Results: The Pg ultrasonicate induced the apoptosis of CD4+ T cells and upregulated the expression of the Th17-associated transcription factor RoRγt and the production of the proinflammatory cytokines IL-17 and IL-6, but downregulated the expression of the essential Treg transcription factor Foxp3 and the production of the anti-inflammatory factors TGF-β and IL-10 via the TLR4 pathway. However, LGG extract maintained Th17/Treg homeostasis by decreasing the IL-17+ Th17 proportion and increasing the CD25+ Foxp3+ Treg proportion via the TLR2 pathway. In vivo, Pg-stimulated CD4+ T cells aggravated DSS-induced colitis by increasing the Th17/Treg ratio in the colon and lamina propria lymphocytes (LPLs), and Pg + LGG-stimulated CD4+ T cells relieved colitis by decreasing the Th17/Treg ratio via the JAK-STAT signalling pathway.
Conclusions: Our findings suggest that pathogenic Pg and probiotic LGG can directly regulate the Th17/Treg balance via different TLRs.
Keywords: Lactobacillusrhamnosus GG; Porphyromonasgingivalis; Th17; Treg; colitis; toll‐like receptor.
© 2020 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lin X, Sun Q, Zhou L et al Colonic epithelial mTORC1 promotes ulcerative colitis through COX‐2‐mediated Th17 responses. Mucosal Immunol 2018; 11: 1663–1673. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
