Spatial Heterogeneity in Particle-Associated, Light-Independent Superoxide Production Within Productive Coastal Waters
- PMID: 33282615
- PMCID: PMC7685101
- DOI: 10.1029/2020JC016747
Spatial Heterogeneity in Particle-Associated, Light-Independent Superoxide Production Within Productive Coastal Waters
Abstract
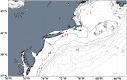
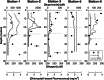
In the marine environment, the reactive oxygen species (ROS) superoxide is produced through a diverse array of light-dependent and light-independent reactions, the latter of which is thought to be primarily controlled by microorganisms. Marine superoxide production influences organic matter remineralization, metal redox cycling, and dissolved oxygen concentrations, yet the relative contributions of different sources to total superoxide production remain poorly constrained. Here we investigate the production, steady-state concentration, and particle-associated nature of light-independent superoxide in productive waters off the northeast coast of North America. We find exceptionally high levels of light-independent superoxide in the marine water column, with concentrations ranging from 10 pM to in excess of 2,000 pM. The highest superoxide concentrations were particle associated in surface seawater and in aphotic seawater collected meters off the seafloor. Filtration of seawater overlying the continental shelf lowered the light-independent, steady-state superoxide concentration by an average of 84%. We identify eukaryotic phytoplankton as the dominant particle-associated source of superoxide to these coastal waters. We contrast these measurements with those collected at an off-shelf station, where superoxide concentrations did not exceed 100 pM, and particles account for an average of 40% of the steady-state superoxide concentration. This study demonstrates the primary role of particles in the production of superoxide in seawater overlying the continental shelf and highlights the importance of light-independent, dissolved-phase reactions in marine ROS production.
Keywords: extracellular superoxide; light‐independent ROS; reactive oxygen species.
©2020. The Authors.
Figures



References
-
- Armoza‐Zvuloni, R. , & Shaked, Y. (2014). Release of hydrogen peroxide and antioxidants by the coral Stylophora pistillata to its external milieu. Biogeosciences, 11(17), 4587–4598. 10.5194/bg-11-4587-2014 - DOI
