Enteric Glia Play a Critical Role in Promoting the Development of Colorectal Cancer
- PMID: 33282743
- PMCID: PMC7691584
- DOI: 10.3389/fonc.2020.595892
Enteric Glia Play a Critical Role in Promoting the Development of Colorectal Cancer
Abstract
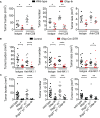
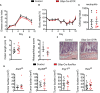
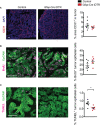
Enteric glia are a distinct population of peripheral glial cells in the enteric nervous system that regulate intestinal homeostasis, epithelial barrier integrity, and gut defense. Given these unique attributes, we investigated the impact of enteric glia depletion on tumor development in azoxymethane/dextran sodium sulfate (AOM/DSS)-treated mice, a classical model of colorectal cancer (CRC). Depleting GFAP+ enteric glia resulted in a profoundly reduced tumor burden in AOM/DSS mice and additionally reduced adenomas in the ApcMin /+ mouse model of familial adenomatous polyposis, suggesting a tumor-promoting role for these cells at an early premalignant stage. This was confirmed in further studies of AOM/DSS mice, as enteric glia depletion did not affect the properties of established malignant tumors but did result in a marked reduction in the development of precancerous dysplastic lesions. Surprisingly, the protective effect of enteric glia depletion was not dependent on modulation of anti-tumor immunity or intestinal inflammation. These findings reveal that GFAP+ enteric glia play a critical pro-tumorigenic role during early CRC development and identify these cells as a potential target for CRC prevention.
Keywords: ApcMin/+; azoxymethane/dextran sodium sulfate; colorectal cancer; enteric glia; enteric nervous system.
Copyright © 2020 Yuan, Bhattacharya, Kenkel, Shen, DiMaio, Bagchi, Prestwood, Habtezion and Engleman.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

