Vasculitis in Cystic Fibrosis
- PMID: 33282799
- PMCID: PMC7690646
- DOI: 10.3389/fped.2020.585275
Vasculitis in Cystic Fibrosis
Abstract
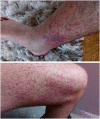
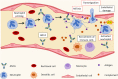
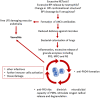
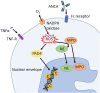
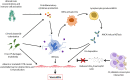
Cystic fibrosis (CF) is an autosomal-recessive multi-organ disease characterized by airways obstruction, recurrent infections, and systemic inflammation. Vasculitis is a severe complication of CF that affects 2-3% of CF patients and is generally associated with poor prognosis. Various pathogenic mechanisms may be involved in the development of CF-related vasculitis. Bacterial colonization leads to persistent activation of neutrophilic granulocytes, inflammation and damage, contributing to the production of antineutrophil cytoplasmic autoantibodies (ANCAs). The presence of ANCA may on the other hand predispose to bacterial colonization and infection, likely entertaining a vicious circle amplifying inflammation and damage. As a result, in CF-associated vasculitis, ongoing inflammation, immune cell activation, the presence of pathogens, and the use of numerous medications may lead to immune complex formation and deposition, subsequently causing leukocytoclastic vasculitis. Published individual case reports and small case series suggest that patients with CF-associated vasculitis require immune modulating treatment, including non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, hydroxychloroquine, and/or disease-modifying anti-rheumatic drugs (DMARDs). As immunosuppression increases the risk of infection and/or malignancy, which are both already increased in people with CF, possible alternative medications may involve the blockade of individual cytokine or inflammatory pathways, or the use of novel CFTR modulators. This review summarizes molecular alterations involved in CF-associated vasculitis, clinical presentation, and complications, as well as currently available and future treatment options.
Keywords: cystic fibrosis; damage; immune complex; inflammation; pathophysiology; treatment; vasculitis.
Copyright © 2020 Sposito, McNamara and Hedrich.
Figures

References
-
- National Library of Medicine (US) Cystic Fibrosis. Genetics Home Reference. (2020) Available online at: https://ghr.nlm.nih.gov/condition/cystic-fibrosis (accessed September 22, 2020).
-
- Cystic Fibrosis Mutation Database. Available online at: http://www.genet.sickkids.on.ca/Home.html (accessed March 20, 2020).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

