The Role of ADF/Cofilin in Synaptic Physiology and Alzheimer's Disease
- PMID: 33282872
- PMCID: PMC7688896
- DOI: 10.3389/fcell.2020.594998
The Role of ADF/Cofilin in Synaptic Physiology and Alzheimer's Disease
Abstract
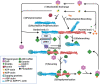
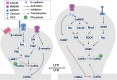
Actin-depolymerization factor (ADF)/cofilin, a family of actin-binding proteins, are critical for the regulation of actin reorganization in response to various signals. Accumulating evidence indicates that ADF/cofilin also play important roles in neuronal structure and function, including long-term potentiation and depression. These are the most extensively studied forms of long-lasting synaptic plasticity and are widely regarded as cellular mechanisms underlying learning and memory. ADF/cofilin regulate synaptic function through their effects on dendritic spines and the trafficking of glutamate receptors, the principal mediator of excitatory synaptic transmission in vertebrates. Regulation of ADF/cofilin involves various signaling pathways converging on LIM domain kinases and slingshot phosphatases, which phosphorylate/inactivate and dephosphorylate/activate ADF/cofilin, respectively. Actin-depolymerization factor/cofilin activity is also regulated by other actin-binding proteins, activity-dependent subcellular distribution and protein translation. Abnormalities in ADF/cofilin have been associated with several neurodegenerative disorders such as Alzheimer's disease. Therefore, investigating the roles of ADF/cofilin in the brain is not only important for understanding the fundamental processes governing neuronal structure and function, but also may provide potential therapeutic strategies to treat brain disorders.
Keywords: ADF/cofilin; AMPA glutamate receptor; LTD; LTP; dendritic spine.
Copyright © 2020 Ben Zablah, Merovitch and Jia.
Figures
References
-
- Agrawal P. B., Joshi M., Savic T., Chen Z., Beggs A. H. (2012). Normal myofibrillar development followed by progressive sarcomeric disruption with actin accumulations in a mouse Cfl2 knockout demonstrates requirement of cofilin-2 for muscle maintenance. Hum. Mol. Genet. 21 2341–2356. 10.1093/hmg/dds053 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

